Introduction to Organic chemistry
Organic chemistry is the branch of chemistry that studies carbon compounds present in living things, once living things or synthetic/man-made.
Compounds that makes up living things whether alive or dead mainly contain carbon. Carbon is tetravalent.
It is able to form stable covalent bonds with itself and many non-metals like hydrogen, nitrogen ,oxygen and halogens to form a variety of compounds. This is because:
(i) carbon uses all the four valence electrons to form four strong covalent bond.
(ii)carbon can covalently bond to form a single, double or triple covalent bond with itself.
(iii)carbon atoms can covalently bond to form a very long chain or ring.
When carbon covalently bond with Hydrogen, it forms a group of organic compounds called Hydrocarbons
A.HYDROCARBONS (HCs)
Hydrocarbons are a group of organic compounds containing /made up of hydrogen and carbon atoms only.
Depending on the type of bond that exist between the individual carbon atoms, hydrocarbon are classified as:
(i) Alkanes
(ii) Alkenes
(iii) Alkynes
(i) Alkanes
(a)Nomenclature/Naming
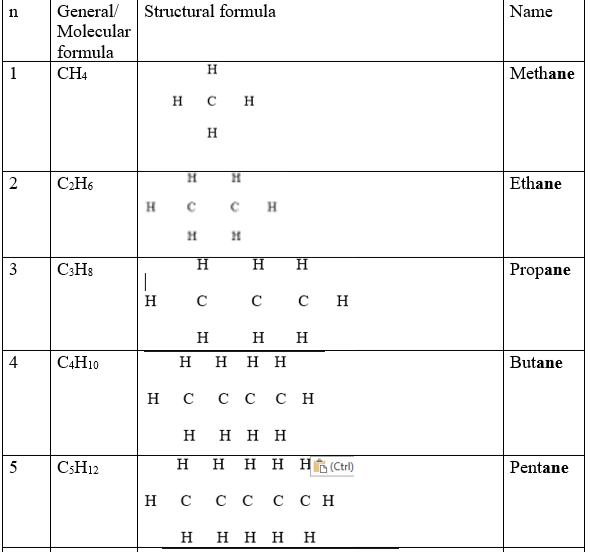
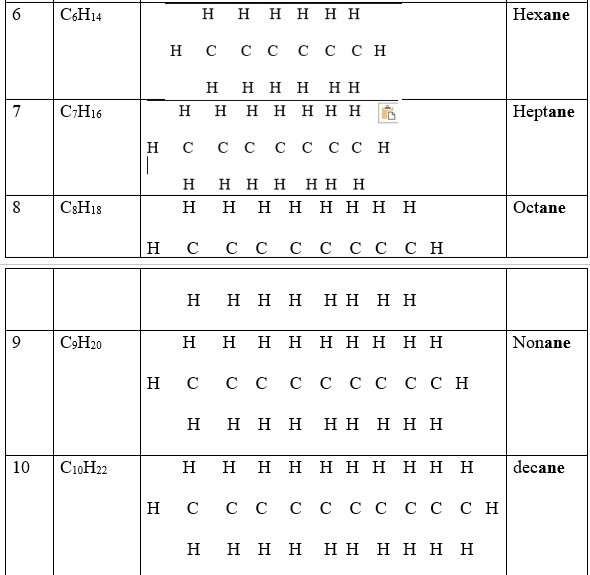
These are hydrocarbons with a general formula CnH2n+2 where n is the number of Carbon atoms in a molecule.
The carbon atoms are linked by single bond to each other and to hydrogen atoms.
They include:
Note
1.The general formula/molecular formular of a compound shows the number of each atoms of elements making the compound e.g.
Decane has a general/molecular formula C10H22 ;this means there are 10 carbon atoms and 22 hydrogen atoms in a molecule of decane.
2.The structural formula shows the arrangement/bonding of atoms of each element making the compound e.g
Decane has the structural formula as in the table above ;this means the 1st carbon from left to right is bonded to three hydrogen atoms and one carbon atom.
The 2nd carbon atom is joined/bonded to two other carbon atoms and two Hydrogen atoms.
3.Since carbon is tetravalent ,each atom of carbon in the alkane MUST always be bonded using four covalent bond /four shared pairs of electrons.
4.Since Hydrogen is monovalent ,each atom of hydrogen in the alkane MUST always be bonded using one covalent bond/one shared pair of electrons.
5.One member of the alkane differ from the next/previous by a CH2 group.
e.g
Propane differ from ethane by one carbon and two Hydrogen atoms form ethane. Ethane differ from methane also by one carbon and two Hydrogen atoms
6.A group of compounds that differ by a CH2 group from the next /previous consecutively is called a homologous series.
7.A homologous series:
(i) differ by a CH2 group from the next /previous consecutively
(ii)have similar chemical properties
(iii)have similar chemical formula that can be represented by a general formula e.g alkanes have the general formulaCnH2n+2.
(iv)the physical properties (e.g.melting/boiling points)show steady gradual change)
8.The 1st four alkanes have the prefix meth_,eth_,prop_ and but_ to represent 1,2,3 and 4 carbons in the compound. All other use the numeral prefix pent_,Hex_,hept_ , etc to show also the number of carbon atoms.
9.If one hydrogen atom in an alkane is removed, an alkyl group is formed.e.g
| Alkane name | molecular structure CnH2n+2 | Alkyl name | Molecula structure CnH2n+1 |
| methane | CH4 | methyl | CH3 |
| ethane | CH3CH3 | ethyl | CH3 CH2 |
| propane | CH3 CH2 CH3 | propyl | CH3 CH2 CH2 |
| butane | CH3 CH2 CH2 CH3 | butyl | CH3 CH2 CH2 CH2 |
(b)Isomers of alkanes
Isomers are compounds with the same molecular general formula but different molecular structural formula.
Isomerism is the existence of a compounds having the same general/molecular formula but different structural formula.
The 1st three alkanes do not form isomers.Isomers are named by using the IUPAC(International Union of Pure and Applied Chemistry) system of nomenclature/naming.
The IUPAC system of nomenclatureuses the following basic rules/guidelines:
1.Identify the longest continuous carbon chain to get/determine the parent alkane.
2.Number the longest chain form the end of the chain that is near the branches so as the branch get the lowest number possible
3. Determine the position, number and type of branches. Name them as methyl, ethyl, propyl e.tc. according to the number of carbon chains attached to the parent alkane. Name them fluoro-,chloro-,bromo-,iodo- if they are halogens
4.Use prefix di-,tri-,tetra-,penta-,hexa- to show the number of branches attached to the parent alkane.
Practice on IUPAC nomenclature of alkanes
(a)Draw the structure of:
(i)2-methylpentane
Procedure
1. Identify the longest continuous carbon chain to get/determine the parent alkane.
Butane is the parent name CH3 CH2 CH2 CH3
2. Number the longest chain form the end of the chain that is near the branches so as the branch get the lowest number possible
The methyl group is attached to Carbon “2”
3. Determine the position, number and type of branches. Name them as methyl, ethyl, propyl e.tc. according to the number of carbon chains attached to the parent alkane i.e
Position of the branch at carbon “2”
Number of branches at carbon “1”
Type of the branch “methyl” hence
Molecular formula
CH3
CH3 CH CH2 CH3 //CH3 CH (CH3 ) CH2CH3
Structural formula
H H H H
H C C C C H
H H H
H C H
H
(ii)2,2-dimethylpentane
Procedure
1. Identify the longest continuous carbon chain to get/determine the parent alkane.
Butane is the parent name CH3 CH2 CH2 CH3
2. Number the longest chain form the end of the chain that is near the branches so as the branch get the lowest number possible
The methyl group is attached to Carbon “2”
3. Determine the position, number and type of branches. Name them as methyl, ethyl, propyl e.tc. according to the number of carbon chains attached to the parent alkane i.e
Position of the branch at carbon “2”
Number of branches at carbon “2”
Type of the branch two“methyl” hence
Molecular formular
(iii) 2,2,3-trimethylbutane
Procedure
1. Identify the longest continuous carbon chain to get/determine the parent alkane.
Butane is the parent name CH3 CH2 CH2 CH3
2. Number the longest chain form the end of the chain that is near the branches so as the branch get the lowest number possible
The methyl group is attached to Carbon “2 and 3”
3. Determine the position, number and type of branches. Name them as methyl, ethyl, propyl e.tc. according to the number of carbon chains attached to the parent alkane i.e
Position of the branch at carbon “2 and 3”
Number of branches at carbon “3”
Type of the branch three “methyl” hence
Molecular formular
CH3
CH3 C CH CH3 //CH3 C (CH3 )3 CH2CH3
CH3 CH3
(iv) 1,1,1,2,2,2-hexabromoethane
Molecular formula
CBr3 CBr3
(v) 1,1,1-tetrachloro-2,2-dimethylbutane
CH3
CCl 3 C CH3 // C Cl 3 C (CH3 )2 CH3
CH3
