ALKANOIC ACIDS (Carboxylic acids)
Alkanoic acids belong to a homologous series of organic compounds with a general formula CnH2n +1 COOH and thus -COOH as the functional group .The 1st ten alkanoic acids include:
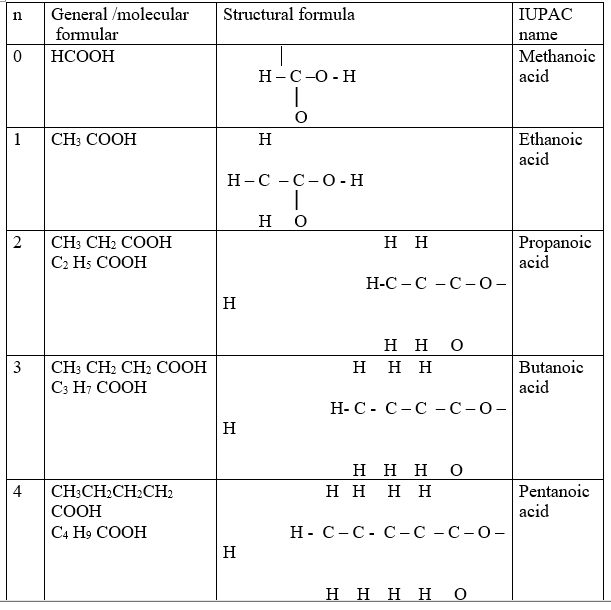
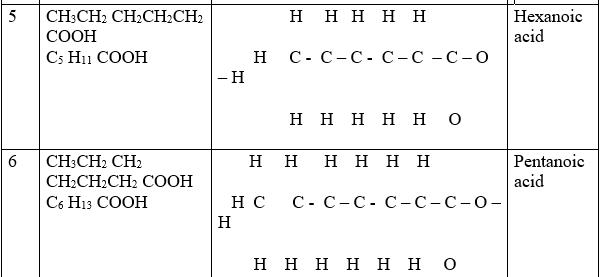
Alkanoic acids like alkanols /alkanes/alkenes/alkynes form a homologous series where:
(i)the general name of an alkanoic acids is derived from the alkane name then ending with “–oic” acid as the table above shows.
(ii) the members have R-COOH/R C-O-H as the functional group.
O
(iii)they have the same general formula represented by R-COOH where R is an alkyl group.
(iv)each member differ by –CH2– group from the next/previous.
(v)they show a similar and gradual change in their physical properties e.g. boiling and melting point.
(vi)they show similar and gradual change in their chemical properties.
(vii) since they are acids they show similar properties with mineral acids.
(B) ISOMERS OF ALKANOIC ACIDS.
lkanoic acids exhibit both structural and position isomerism. The isomers are named by using the following basic guidelines
(i)Like alkanes. identify the longest carbon chain to be the parent name.
(ii)Identify the position of the -C-O-H functional group to give it the smallest
O
/lowest position.
(iii)Identify the type and position of the side group branches.
Practice examples on isomers of alkanoic acids
1.Isomers of butanoic acid C3H7COOH
CH3 CH2 CH2 COOH
Butan-1-oic acid
CH3
H2C C COOH 2-methylpropan-1-oic acid
2-methylpropan-1-oic acid and Butan-1-oic acid are structural isomers because the position of the functional group does not change but the arrangement of the atoms in the molecule does.
2.Isomers of pentanoic acid C4H9COOH
CH3CH2CH2CH2 COOH pentan-1-oic acid
CH3
CH3CH2CH COOH 2-methylbutan-1-oic acid
CH3
H3C C COOH 2,2-dimethylpropan-1-oic acid
CH3
3.Ethan-1,2-dioic acid
O O
HOOC- COOH // H – O – C – C – O – H
4.Propan-1,3-dioic acid
O H O
HOOC- CH2COOH // H – O – C – C – C – O – H
H
5.Butan-1,4-dioic acid
O H H O
HOOC CH2 CH2 COOH H- O – C – C – C – C –O – H
H H
6.2,2-dichloroethan-1,2-dioic acid
HOOCCHCl2 Cl
H – O – C – C – Cl
O H
(C) LABORATORY AND INDUSTRIAL PREPARATIONOF ALKANOIC ACIDS.
In a school laboratory, alkanoic acids can be prepared by adding an oxidizing agent (H+/KMnO4 or H+/K2Cr2O7)to the corresponding alkanol then warming.
The oxidation converts the alkanol first to an alkanal the alkanoic acid.
NB Acidified KMnO4 is a stronger oxidizing agent than acidified K2Cr2O7
General equation:
R- CH2 – OH + [O] –H+/KMnO4–> R- CH –O + H2O(l)
(alkanol) (alkanal)
R- CH – O + [O] –H+/KMnO4–> R- C –OOH
(alkanal) (alkanoic acid)
Examples
1.Ethanol on warming in acidified KMnO4 is oxidized to ethanal then ethanoic acid .
CH3– CH2 – OH + [O] –H+/KMnO4–> CH3– CH –O + H2O(l)
(ethanol) (ethanal)
CH3– CH – O + [O] –H+/KMnO4–> CH3– C –OOH
(ethanal) (ethanoic acid)
2Propanol on warming in acidified KMnO4 is oxidized to propanal then propanoic acid
CH3– CH2 CH2 – OH + [O] –H+/KMnO4–> CH3– CH2 CH –O + H2O(l)
(propanol) (propanal)
CH3– CH – O + [O] –H+/KMnO4–> CH3– C –OOH
(propanal) (propanoic acid)
Industrially,large scale manufacture of alkanoic acid like ethanoic acid is obtained from:
(a)Alkenes reacting with steam at high temperatures and pressure in presence of phosphoric(V)acid catalyst and undergo hydrolysis to form alkanols. i.e.
Alkenes + Steam/water — H2PO4 Catalyst–> Alkanol
The alkanol is then oxidized by air at 5 atmosphere pressure with Manganese (II)sulphate(VI) catalyst to form the alkanoic acid.
Alkanol + Air — MnSO4 Catalyst/5 atm pressure–> Alkanoic acid
Example
Ethene is mixed with steam over a phosphoric(V)acid catalyst,300oC temperature and 60 atmosphere pressure to form ethanol.
CH2=CH2 + H2O -> CH3 CH2OH
(Ethene) (Ethanol)
This is the industrial large scale method of manufacturing ethanol
Ethanol is then oxidized by air at 5 atmosphere pressure with Manganese (II)sulphate(VI) catalyst to form the ethanoic acid.
CH3 CH2OH + [O] — MnSO4 Catalyst/5 atm pressure–> CH3 COOH
(Ethanol) (Ethanoic acid)
(b)Alkynes react with liquid water at high temperatures and pressure in presence of Mercury(II)sulphate(VI)catalyst and 30% concentrated sulphuric(VI)acid to form alkanals.
Alkyne + Water — Mercury(II)sulphate(VI)catalyst–> Alkanal
The alkanal is then oxidized by air at 5 atmosphere pressure with Manganese (II) sulphate(VI) catalyst to form the alkanoic acid.
Alkanal + air/oxygen — Manganese(II)sulphate(VI)catalyst–> Alkanoic acid
Example
Ethyne react with liquid water at high temperature and pressure with Mercury (II) sulphate (VI)catalyst and 30% concentrated sulphuric(VI)acid to form ethanal.
CH = CH + H2O –HgSO4–> CH3 CH2O
(Ethyne) (Ethanal)
This is another industrial large scale method of manufacturing ethanol from large quantities of ethyne found in natural gas.
Ethanal is then oxidized by air at 5 atmosphere pressure with Manganese (II)sulphate(VI) catalyst to form the ethanoic acid.
CH3 CH2O + [O] — MnSO4 Catalyst/5 atm pressure–> CH3 COOH
(Ethanal) (Oxygen from air) (Ethanoic acid)
(D) PHYSICAL AND CHEMICAL PROPERTIES OF ALKANOIC ACIDS.
I.Physical properties of alkanoic acids
The table below shows some physical properties of alkanoic acids
| Alkanol | Melting point(oC) | Boiling point(oC) | Density(gcm-3) | Solubility in water |
| Methanoic acid | 18.4 | 101 | 1.22 | soluble |
| Ethanoic acid | 16.6 | 118 | 1.05 | soluble |
| Propanoic acid | -2.8 | 141 | 0.992 | soluble |
| Butanoic acid | -8.0 | 164 | 0.964 | soluble |
| Pentanoic acid | -9.0 | 187 | 0.939 | Slightly soluble |
| Hexanoic acid | -11 | 205 | 0.927 | Slightly soluble |
| Heptanoic acid | -3 | 223 | 0.920 | Slightly soluble |
| Octanoic acid | 11 | 239 | 0.910 | Slightly soluble |
| Nonanoic acid | 16 | 253 | 0.907 | Slightly soluble |
| Decanoic acid | 31 | 269 | 0.905 | Slightly soluble |
From the table note the following:
- Melting and boiling point decrease as the carbon chain increases due to increase in intermolecular forces of attraction between the molecules requiring more energy to separate the molecules.
- The density decreases as the carbon chain increases as the intermolecular forces of attraction increases between the molecules making the molecule very close reducing their volume in unit mass.
- Solubility decreases as the carbon chain increases as the soluble –COOH end is shielded by increasing insoluble alkyl/hydrocarbon chain.
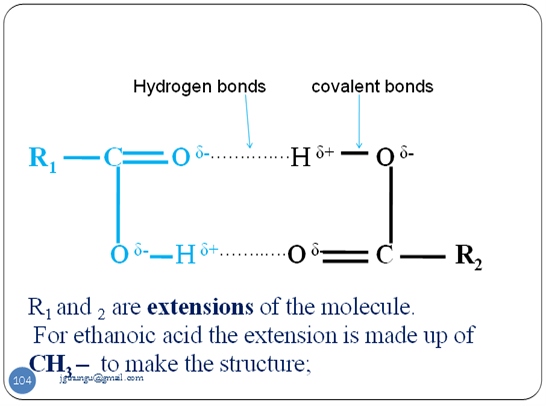
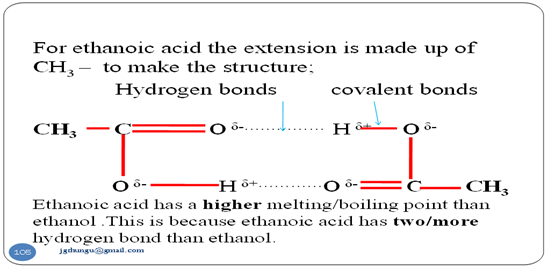
- Like alkanols ,alkanoic acids exist as dimmers due to the hydrogen bonds within the molecule. i.e..
II Chemical properties of alkanoic acids
The following experiments shows the main chemical properties of ethanoic (alkanoic) acid.
(a)Effect on litmus papers
Experiment
Dip both blue and red litmus papers in ethanoic acid. Repeat with a solution of succinic acid, citric acid, oxalic acid, tartaric acid and dilute nitric(V)acid.
Sample observations
| Solution/acid | Observations/effect on litmus papers | Inference |
| Ethanoic acid | Blue litmus paper turn red Red litmus paper remain red | H3O+/H+(aq)ion |
| Succinic acid | Blue litmus paper turn red Red litmus paper remain red | H3O+/H+(aq)ion |
| Citric acid | Blue litmus paper turn red Red litmus paper remain red | H3O+/H+(aq)ion |
| Oxalic acid | Blue litmus paper turn red Red litmus paper remain red | H3O+/H+(aq)ion |
| Tartaric acid | Blue litmus paper turn red Red litmus paper remain red | H3O+/H+(aq)ion |
| Nitric(V)acid | Blue litmus paper turn red Red litmus paper remain red | H3O+/H+(aq)ion |
Explanation
All acidic solutions contains H+/H3O+(aq) ions. The H+ /H3O+ (aq) ions is responsible for turning blue litmus paper/solution to red
(b)pH
Experiment
Place 2cm3 of ethaoic acid in a test tube. Add 2 drops of universal indicator solution and determine its pH. Repeat with a solution of succinic acid, citric acid, oxalic acid, tartaric acid and dilute sulphuric (VI)acid.
Sample observations
| Solution/acid | pH | Inference |
| Ethanoic acid | 4/5/6 | Weakly acidic |
| Succinic acid | 4/5/6 | Weakly acidic |
| Citric acid | 4/5/6 | Weakly acidic |
| Oxalic acid | 4/5/6 | Weakly acidic |
| Tartaric acid | 4/5/6 | Weakly acidic |
| Sulphuric(VI)acid | 1/2/3 | Strongly acidic |
Explanations
Alkanoic acids are weak acids that partially/partly dissociate to release few H+ ions in solution. The pH of their solution is thus 4/5/6 showing they form weakly acidic solutions when dissolved in water.
All alkanoic acid dissociate to releases the “H” at the functional group in -COOH to form the alkanoate ion; –COO–
Mineral acids(Sulphuric(VI)acid, Nitric(V)acid and Hydrochloric acid) are strong acids that wholly/fully dissociate to release many H+ ions in solution. The pH of their solution is thus 1/2/3 showing they form strongly acidic solutions when dissolved in water.i.e
Examples
- CH3COOH(aq) CH3COO–(aq) + H+(aq)
(ethanoic acid) (ethanoate ion) (few H+ ion)
- CH3 CH2COOH(aq) CH3 CH2COO–(aq) + H+(aq)
(propanoic acid) (propanoate ion) (few H+ ion)
- CH3 CH2 CH2COOH(aq) CH3 CH2 CH2COO–(aq) + H+(aq)
(Butanoic acid) (butanoate ion) (few H+ ion)
- HOOH(aq) HOO–(aq) + H+(aq)
(methanoic acid) (methanoate ion) (few H+ ion)
- H2 SO4 (aq) SO42- (aq) + 2H+(aq)
(sulphuric(VI) acid) (sulphate(VI) ion) (many H+ ion)
- HNO3 (aq) NO3– (aq) + H+(aq)
(nitric(V) acid) (nitrate(V) ion) (many H+ ion)
(c)Reaction with metals
Experiment
Place about 4cm3 of ethanoic acid in a test tube. Put about 1cm length of polished magnesium ribbon. Test any gas produced using a burning splint. Repeat with a solution of succinic acid, citric acid, oxalic acid, tartaric acid and dilute sulphuric (VI) acid.
Sample observations
| Solution/acid | Observations | Inference |
| Ethanoic acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that burn with “pop” sound/explosion | H3O+/H+(aq)ion |
| Succinic acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that burn with “pop” sound/explosion | H3O+/H+(aq)ion |
| Citric acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that burn with “pop” sound/explosion | H3O+/H+(aq)ion |
| Oxalic acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that burn with “pop” sound/explosion | H3O+/H+(aq)ion |
| Tartaric acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that burn with “pop” sound/explosion | H3O+/H+(aq)ion |
| Nitric(V)acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that burn with “pop” sound/explosion | H3O+/H+(aq)ion |
Explanation
Metals higher in the reactivity series displace the hydrogen in all acids to evolve/produce hydrogen gas and form a salt. Alkanoic acids react with metals with metals to form alkanoates salt and produce/evolve hydrogen gas .Hydrogen extinguishes a burning splint with a pop sound/explosion. Only the “H”in the functional group -COOH is /are displaced and not in the alkyl hydrocarbon chain.
Alkanoic acid + Metal -> Alkanoate + Hydrogen gas. i.e.
Examples
1. For a monovalent metal with monobasic acid
2R – COOH + 2M -> 2R- COOM + 2H2(g)
2.For a divalent metal with monobasic acid
2R – COOH + M -> (R- COO) 2M + H2(g)
3.For a divalent metal with dibasic acid
HOOC-R-COOH+ M -> MOOC-R-COOM + H2(g)
4.For a monovalent metal with dibasic acid
HOOC-R-COOH+ 2M -> MOOC-R-COOM + H2(g)
5 For mineral acids
(i)Sulphuric(VI)acid is a dibasic acid
H2 SO4 (aq) + 2M -> M2 SO4 (aq) + H2(g)
H2 SO4 (aq) + M -> MSO4 (aq) + H2(g)
(ii)Nitric(V) and hydrochloric acid are monobasic acid
HNO3 (aq) + 2M -> 2MNO3 (aq) + H2(g)
HNO3 (aq) + M -> M(NO3 ) 2 (aq) + H2(g)
Examples
1.Sodium reacts with ethanoic acid to form sodium ethanoate and produce. hydrogen gas.
Caution: This reaction is explosive.
CH3COOH (aq) + Na(s) -> CH3COONa (aq) + H2(g)
(Ethanoic acid) (Sodium ethanoate)
2.Calcium reacts with ethanoic acid to form calcium ethanoate and produce. hydrogen gas.
2CH3COOH (aq) + Ca(s) -> (CH3COO) 2Ca (aq) + H2(g)
(Ethanoic acid) (Calcium ethanoate)
3.Sodium reacts with ethan-1,2-dioic acid to form sodium ethan-1,2-dioate and produce. hydrogen gas.
HOOC-COOH+ 2Na -> NaOOC – COONa + H2(g)
(ethan-1,2-dioic acid) (sodium ethan-1,2-dioate)
Commercial name of ethan-1,2-dioic acid is oxalic acid. The salt is sodium oxalate.
4.Magnesium reacts with ethan-1,2-dioic acid to form magnesium ethan-1,2-dioate and produce. hydrogen gas.
HOOC-R-COOH+ Mg -> ( OOC – COO)Mg + H2(g)
(ethan-1,2-dioic acid) (magnesium ethan-1,2-dioate)
5.Magnesium reacts with
(i)Sulphuric(VI)acid to form Magnesium sulphate(VI)
H2 SO4 (aq) + Mg -> MgSO4 (aq) + H2(g)
(ii)Nitric(V) and hydrochloric acid are monobasic acid
2HNO3 (aq) + Mg -> M(NO3 ) 2 (aq) + H2(g)
(d)Reaction with hydrogen carbonates and carbonates
Experiment
Place about 3cm3 of ethanoic acid in a test tube. Add about 0.5g/ ½ spatula end full of sodium hydrogen carbonate/sodium carbonate. Test the gas produced using lime water. Repeat with a solution of succinic acid, citric acid, oxalic acid, tartaric acid and dilute sulphuric (VI) acid.
Sample observations
| Solution/acid | Observations | Inference |
| Ethanoic acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that forms a white precipitate with lime water | H3O+/H+(aq)ion |
| Succinic acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that forms a white precipitate with lime water | H3O+/H+(aq)ion |
| Citric acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that forms a white precipitate with lime water | H3O+/H+(aq)ion |
| Oxalic acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that forms a white precipitate with lime water | H3O+/H+(aq)ion |
| Tartaric acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that forms a white precipitate with lime water | H3O+/H+(aq)ion |
| Nitric(V)acid | (i)effervescence, fizzing, bubbles (ii)colourless gas produced that forms a white precipitate with lime water | H3O+/H+(aq)ion |
All acids react with hydrogen carbonate/carbonate to form salt ,water and evolve/produce bubbles of carbon(IV)oxide and water.
Carbon(IV)oxide forms a white precipitate when bubbled in lime water/extinguishes a burning splint.
Alkanoic acids react with hydrogen carbonate/carbonate to form alkanoates ,water and evolve/produce bubbles of carbon(IV)oxide and water.
Alkanoic acid + hydrogen carbonate -> alkanoate + water + carbon(IV)oxide
Alkanoic acid + carbonate -> alkanoate + water + carbon(IV)oxide
Examples
- Sodium hydrogen carbonate reacts with ethanoic acid to form sodium ethanoate ,water and carbon(IV)oxide gas.
CH3COOH (aq) + NaHCO3 (s) -> CH3COONa (aq) + H2O(l) + CO2 (g)
(Ethanoic acid) (Sodium ethanoate)
2.Sodium carbonate reacts with ethanoic acid to form sodium ethanoate ,water and carbon(IV)oxide gas.
2CH3COOH (aq) + Na2CO3 (s) -> 2CH3COONa (aq) + H2O(l) + CO2 (g)
(Ethanoic acid) (Sodium ethanoate)
3.Sodium carbonate reacts with ethan-1,2-dioic acid to form sodium ethanoate ,water and carbon(IV)oxide gas.
HOOC-COOH+ Na2CO3 (s) -> NaOOC – COONa + H2O(l) + CO2 (g)
(ethan-1,2-dioic acid) (sodium ethan-1,2-dioate)
4.Sodium hydrogen carbonate reacts with ethan-1,2-dioic acid to form sodium ethanoate ,water and carbon(IV)oxide gas.
HOOC-COOH+ 2NaHCO3 (s) -> NaOOC – COONa + H2O(l) + 2CO2 (g)
(ethan-1,2-dioic acid) (sodium ethan-1,2-dioate)
(e)Esterification
Experiment
Place 4cm3 of ethanol acid in a boiling tube.
Add equal volume of ethanoic acid. To the mixture, add 2 drops of concentrated sulphuric(VI)acid carefully. Warm/heat gently on Bunsen flame.
Pour the mixture into a beaker containing 50cm3 of water. Smell the products. Repeat with a solution of succinic acid, citric acid, oxalic acid, tartaric acid and dilute sulphuric (VI) acid.
Sample observations
| Solution/acid | Observations |
| Ethanoic acid | Sweet fruity smell |
| Succinic acid | Sweet fruity smell |
| Citric acid | Sweet fruity smell |
| Oxalic acid | Sweet fruity smell |
| Tartaric acid | Sweet fruity smell |
| Dilute sulphuric(VI)acid | No sweet fruity smell |
Explanation
Alkanols react with alkanoic acid to form the sweet smelling homologous series of esters and water.The reaction is catalysed by concentrated sulphuric(VI)acid in the laboratory but naturally by sunlight /heat.Each ester has a characteristic smell derived from the many possible combinations of alkanols and alkanoic acids.
Alkanol + Alkanoic acids -> Ester + water
Esters derive their names from the alkanol first then alkanoic acids. The alkanol “becomes” an alkyl group and the alkanoic acid “becomes” alkanoate hence alkylalkanoate. e.g.
Ethanol + Ethanoic acid -> Ethylethanoate + Water
Ethanol + Propanoic acid -> Ethylpropanoate + Water
Ethanol + Methanoic acid -> Ethylmethanoate + Water
Ethanol + butanoic acid -> Ethylbutanoate + Water
Propanol + Ethanoic acid -> Propylethanoate + Water
Methanol + Ethanoic acid -> Methyethanoate + Water
Methanol + Decanoic acid -> Methyldecanoate + Water
Decanol + Methanoic acid -> Decylmethanoate + Water
During the formation of the ester, the “O” joining the alkanol and alkanoic acid comes from the alkanol.
R1 -COOH + R2 –OH -> R1 -COO –R2 + H2O
Examples
1. Ethanol reacts with ethanoic acid to form the ester ethyl ethanoate and water.
Ethanol + Ethanoic acid –Conc. H2SO4 –>Ethylethanoate + Water
C2H5OH (l) + CH3COOH(l) –Conc. H2SO4 –> CH3COO C2H5(aq) +H2O(l)
CH3CH2OH (l)+ CH3COOH(l) –Conc. H2SO4 –> CH3COOCH2CH3(aq) +H2O(l)
2. Ethanol reacts with propanoic acid to form the ester ethylpropanoate and water.
Ethanol + Propanoic acid –Conc. H2SO4 –>Ethylethanoate + Water
C2H5OH (l)+ CH3 CH2COOH(l) –Conc. H2SO4 –>CH3CH2COO C2H5(aq) +H2O(l)
CH3CH2OH (l)+ CH3 CH2COOH(l) –Conc. H2SO4 –>
CH3 CH2COOCH2CH3(aq) +H2O(l)
3. Methanol reacts with ethanoic acid to form the ester methyl ethanoate and water.
Methanol + Ethanoic acid –Conc. H2SO4 –>Methylethanoate + Water
CH3OH (l) + CH3COOH(l) –Conc. H2SO4 –> CH3COO CH3(aq) +H2O(l)
4. Methanol reacts with propanoic acid to form the ester methyl propanoate and water.
Methanol + propanoic acid –Conc. H2SO4 –>Methylpropanoate + Water
CH3OH (l)+ CH3 CH2COOH(l) –Conc. H2SO4 –> CH3 CH2COO CH3(aq) +H2O(l)
5. Propanol reacts with propanoic acid to form the ester propylpropanoate and water.
Propanol + Propanoic acid –Conc. H2SO4 –>Ethylethanoate + Water
C3H7OH (l)+ CH3 CH2COOH(l) –Conc. H2SO4 –>CH3CH2COO C3H7(aq) +H2O(l)
CH3CH2 CH2OH (l)+ CH3 CH2COOH(l) –Conc. H2SO4 –> CH3 CH2COOCH2 CH2CH3(aq) +H2O(l)
C. DETERGENTS
Detergents are cleaning agents that improve the cleaning power /properties of water.A detergent therefore should be able to:
(i)dissolve substances which water cannot e.g grease ,oil, fat
(ii)be washed away after cleaning.
There are two types of detergents:
(a)Soapy detergents
(b)Soapless detergents
- SOAPY DETERGENTS
Soapy detergents usually called soap is long chain salt of organic alkanoic acids.Common soap is sodium octadecanoate .It is derived from reacting concentrated sodium hydroxide solution with octadecanoic acid(18 carbon alkanoic acid) i.e.
Sodium hydroxide + octadecanoic acid -> Sodium octadecanoate + water
NaOH(aq) + CH3 (CH2) 16 COOH(aq) -> CH3 (CH2) 16 COO – Na+ (aq) +H2 O(l)
Commonly ,soap can thus be represented ;
R-COO – Na+ where;
R is a long chain alkyl group and -COO – Na+ is the alkanoate ion.
In a school laboratory and at industrial and domestic level,soap is made by reacting concentrated sodium hydroxide solution with esters from (animal) fat and oil. The process of making soap is called saponification. During saponification ,the ester is hydrolyzed by the alkali to form sodium salt /soap and glycerol/propan-1,2,3-triol is produced.
Fat/oil(ester)+sodium/potassium hydroxide->sodium/potassium salt(soap)+ glycerol
Fats/Oils are esters with fatty acids and glycerol parts in their structure;
C17H35COOCH2
C17H35COOCH
C17H35COOCH2
When boiled with concentrated sodium hydroxide solution NaOH;
(i)NaOH ionizes/dissociates into Na+ and OH– ions
(ii)fat/oil split into three C17H35COO– and one CH2 CH CH2
(iii) the three Na+ combine with the three C17H35COO– to form the salt C17H35COO– Na+
(iv)the three OH–ions combine with the CH2 CH CH2 to form an alkanol with three functional groups CH2 OH CH OH CH2 OH(propan-1,2,3-triol)
C17H35COOCH2 CH2OH
C17H35COOCH +NaOH -> 3 C17H35COO– Na+ + CHOH
C17H35COOCH2 CH2OH
Ester Alkali Soap glycerol
Generally:
CnH2n+1COOCH2 CH2OH
CnH2n+1COOCH +NaOH -> 3 CnH2n+1COO– Na+ + CHOH
CnH2n+1COOCH2 CH2OH
Ester Alkali Soap glycerol
R – COOCH2 CH2OH
R – COOCH +NaOH -> 3R-COO– Na+ + CHOH
R- COOCH2 CH2OH
Ester Alkali Soap glycerol
During this process a little sodium chloride is added to precipitate the soap by reducing its solubility. This is called salting out.
The soap is then added colouring agents ,perfumes and herbs of choice.
School laboratory preparation of soap
Place about 40 g of fatty (animal fat)beef/meat in 100cm3 beaker .Add about 15cm3 of 4.0M sodium hydroxide solution. Boil the mixture for about 15minutes.Stir the mixture .Add about 5.0cm3 of distilled water as you boil to make up for evaporation. Boil for about another 15minutes.Add about four spatula end full of pure sodium chloride crystals. Continue stirring for another five minutes. Allow to cool. Filter of /decant and wash off the residue with distilled water .Transfer the clean residue into a dry beaker. Preserve.
The action of soap
Soapy detergents:
(i)act by reducing the surface tension of water by forming a thin layer on top of the water.
(ii)is made of a non-polar alkyl /hydrocarbon tail and a polar -COO–Na+ head. The non-polar alkyl /hydrocarbon tail is hydrophobic (water hating) and thus does not dissolve in water .It dissolves in non-polar solvent like grease, oil and fat. The polar -COO–Na+ head is hydrophilic (water loving)and thus dissolve in water. When washing with soapy detergent, the non-polar tail of the soapy detergent surround/dissolve in the dirt on the garment /grease/oil while the polar head dissolve in water.
Through mechanical agitation/stirring/sqeezing/rubbing/beating/kneading, some grease is dislodged/lifted of the surface of the garment. It is immediately surrounded by more soap molecules It float and spread in the water as tiny droplets that scatter light in form of emulsion making the water cloudy and shinny. It is removed from the garment by rinsing with fresh water. The repulsion of the soap head prevent /ensure the droplets do not mix. Once removed, the dirt molecules cannot be redeposited back because it is surrounded by soap molecules.
Advantages and disadvantages of using soapy detergents
Soapy detergents are biodegradable. They are acted upon by bacteria and rot. They thus do not cause environmental pollution.
Soapy detergents have the disadvantage in that:
(i)they are made from fat and oils which are better eaten as food than make soap.
(ii)forms an insoluble precipitate with hard water called scum. Scum is insoluble calcium octadecanoate and Magnesium octadecanoate formed when soap reacts with Ca2+ and Mg2+ present in hard water.
Chemical equation
2C17H35COO– Na+ (aq) + Ca2+(aq) -> (C17H35COO– )Ca2+ (s) + 2Na+(aq)
(insoluble Calcium octadecanote/scum)
2C17H35COO– Na+ (aq) + Mg2+(aq) -> (C17H35COO– )Mg2+ (s) + 2Na+(aq)
(insoluble Magnesium octadecanote/scum)
This causes wastage of soap.
Potassium soaps are better than Sodium soap. Potassium is more expensive than sodium and thus its soap is also more expensive.
(b)SOAPLESS DETERGENTS
Soapless detergent usually called detergent is a long chain salt fromed from by-products of fractional distillation of crude oil.Commonly used soaps include:
(i)washing agents
(ii)toothpaste
(iii)emulsifiers/wetting agents/shampoo
Soapless detergents are derived from reacting:
(i)concentrated sulphuric(VI)acid with a long chain alkanol e.g. Octadecanol(18 carbon alkanol) to form alkyl hydrogen sulphate(VI)
Alkanol + Conc sulphuric(VI)acid -> alkyl hydrogen sulphate(VI) + Water
R –OH + H2SO4 -> R –O-SO3H + H2O
(ii)the alkyl hydrogen sulphate(VI) is then neutralized with sodium/potassium hydroxide to form sodium/potassium alkyl hydrogen sulphate(VI)
Sodium/potassium alkyl hydrogen sulphate(VI) is the soapless detergent.
alkyl hydrogen + Potassium/sodium -> Sodium/potassium + Water
sulphate(VI) hydroxide alkyl hydrogen sulphate(VI)
R –O-SO3H + NaOH -> R –O-SO3– Na+ + H2O
Example
Step I : Reaction of Octadecanol with Conc.H2SO4
C17H35CH2OH(aq) + H2SO4 -> C17H35CH2–O- SO3– H+(aq) + H2O(l)
octadecanol + sulphuric(VI)acid -> Octadecyl hydrogen sulphate(VI) + water
Step II: Neutralization by an alkali
C17H35CH2–O- SO3– H+(aq) + NaOH -> C17H35CH2–O- SO3– Na+(aq) + H2O(l)
Octadecyl hydrogen + sodium/potassium -> sodium/potassium octadecyl+Water
sulphate(VI) hydroxide hydrogen sulphate(VI)
School laboratory preparation of soapless detergent
Place about 20g of olive oil in a 100cm3 beaker. Put it in a trough containing ice cold water.
Add dropwise carefully 18M concentrated sulphuric(VI)acid stirring continuously into the olive oil until the oil turns brown.Add 30cm3 of 6M sodium hydroxide solution.Stir.This is a soapless detergent.
The action of soapless detergents
The action of soapless detergents is similar to that of soapy detergents.The soapless detergents contain the hydrophilic head and a long hydrophobic tail. i.e.
vvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvv-COO–Na+
vvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvv-O-SO3– Na+
(long hydrophobic /non-polar alkyl tail) (hydrophilic/polar/ionic head)
The tail dissolves in fat/grease/oil while the ionic/polar/ionic head dissolves in water.
The tail stick to the dirt which is removed by the attraction of water molecules and the polar/ionic/hydrophilic head by mechanical agitation /squeezing/kneading/ beating/rubbing/scrubbing/scatching.
The suspended dirt is then surrounded by detergent molecules and repulsion of the anion head preventing the dirt from sticking on the material garment.
The tiny droplets of dirt emulsion makes the water cloudy. On rinsing the cloudy emulsion is washed away.
Advantages and disadvantages of using soapless detergents
Soapless detergents are non-biodegradable unlike soapy detergents.
They persist in water during sewage treatment by causing foaming in rivers ,lakes and streams leading to marine /aquatic death.
Soapless detergents have the advantage in that they:
(i)do not form scum with hard water.
(ii)are cheap to manufacture/buying
(iii)are made from petroleum products but soapis made from fats/oil for human consumption.
