Crude oil ,natural gas and biogas are the main sources of alkanes:
(i)Natural gas is found on top of crude oil deposits and consists mainly of methane.
(ii)Biogas is formed from the decay of waste organic products like animal dung and cellulose. When the decay takes place in absence of oxygen , 60-75% by volume of the gaseous mixture of methane gas is produced.
(iii)Crude oil is a mixture of many flammable hydrocarbons/substances. Using fractional distillation, each hydrocarbon fraction can be separated from the other. The hydrocarbon with lower /smaller number of carbon atoms in the chain have lower boiling point and thus collected first.
As the carbon chain increase, the boiling point, viscosity (ease of flow) and colour intensity increase as flammability decrease. Hydrocarbons in crude oil are not pure. They thus have no sharp fixed boiling point.
Uses of different crude oil fractions
| Carbon atoms in a molecule | Common name of fraction | Uses of fraction |
| 1-4 | Gas | L.P.G gas for domestic use |
| 5-12 | Petrol | Fuel for petrol engines |
| 9-16 | Kerosene/Paraffin | Jet fuel and domestic lighting/cooking |
| 15-18 | Light diesel | Heavy diesel engine fuel |
| 18-25 | Diesel oil | Light diesel engine fuel |
| 20-70 | Lubricating oil | Lubricating oil to reduce friction. |
| Over 70 | Bitumen/Asphalt | Tarmacking roads |
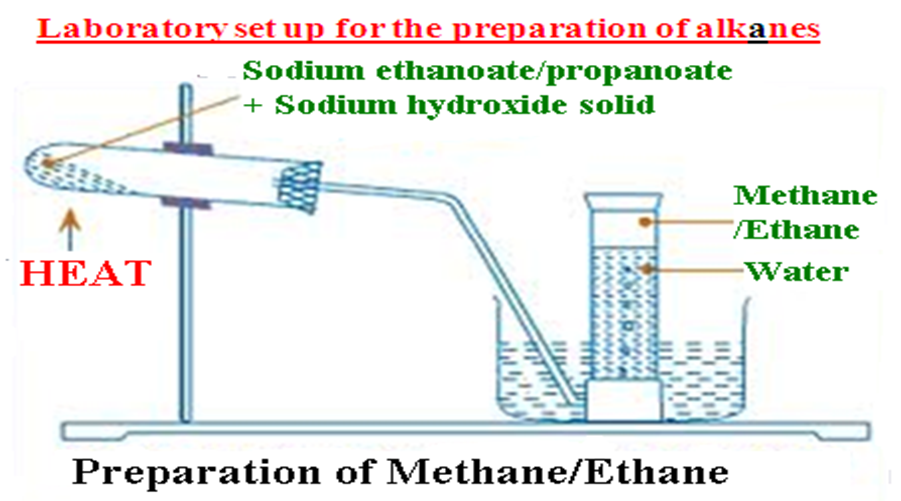
(d)School laboratory preparation of alkanes
In a school laboratory, alkanes may be prepared from the reaction of a sodium alkanoate with solid sodium hydroxide/soda lime.
Chemical equation:
Sodium alkanoate + soda lime -> alkane + Sodium carbonate
CnH2n+1COONa(s) + NaOH(s) -> C n H2n+2 + Na2CO3(s)
The “H” in NaOH is transferred/moves to the CnH2n+1in CnH2n+1COONa(s) to form C n H2n+2.
Examples
1. Methane is prepared from the heating of a mixture of sodium ethanoate and soda lime and collecting over water
Sodium ethanoate + soda lime -> methane + Sodium carbonate
CH3COONa(s) + NaOH(s) -> CH4 + Na2CO3(s)
The “H” in NaOH is transferred/moves to the CH3in CH3COONa(s) to form CH4.
2. Ethane is prepared from the heating of a mixture of sodium propanoate and soda lime and collecting over water
Sodium propanoate + soda lime -> ethane + Sodium carbonate
CH3 CH2COONa(s) + NaOH(s) -> CH3 CH3 + Na2CO3(s)
The “H” in NaOH is transferred/moves to the CH3 CH2in CH3 CH2COONa (s) to form CH3 CH3
3. Propane is prepared from the heating of a mixture of sodium butanoate and soda lime and collecting over water
Sodium butanoate + soda lime -> propane + Sodium carbonate
CH3 CH2CH2COONa(s) + NaOH(s) -> CH3 CH2CH3 + Na2CO3(s)
The “H” in NaOH is transferred/moves to the CH3 CH2 CH2in CH3 CH2CH2COONa (s) to form CH3 CH2CH3
4. Butane is prepared from the heating of a mixture of sodium pentanoate and soda lime and collecting over water
Sodium pentanoate + soda lime -> butane + Sodium carbonate
CH3 CH2 CH2CH2COONa(s)+NaOH(s) -> CH3 CH2CH2CH3 + Na2CO3(s)
The “H” in NaOH is transferred/moves to the CH3CH2 CH2 CH2in CH3 CH2CH2 CH2COONa (s) to form CH3 CH2 CH2CH3
Laboratory set up for the preparation of alkanes
(d)Properties of alkanes
I. Physical properties
Alkanes are colourless gases, solids and liquids that are not poisonous.
They are slightly soluble in water.
The solubility decrease as the carbon chain and thus the molar mass increase
The melting and boiling point increase as the carbon chain increase.
This is because of the increase in van-der-waals /intermolecular forces as the carbon chain increase.
The 1st four straight chain alkanes (methane,ethane,propane and butane)are therefore gases ,the nect six(pentane ,hexane, heptane,octane,nonane, and decane) are liquids while the rest from unidecane(11 carbon atoms) are solids .
The density of straight chain alkanes increase with increasing carbon chain as the intermolecular forces increases.
This reduces the volume occupied by a given mass of the compound.
Summary of physical properties of alkanes
| Alkane | General formula | Melting point(K) | Boiling point(K) | Density gcm-3 | State at room(298K) temperature and pressure atmosphere (101300Pa) |
| Methane | CH4 | 90 | 112 | 0.424 | gas |
| Ethane | CH3CH3 | 91 | 184 | 0.546 | gas |
| Propane | CH3CH2CH3 | 105 | 231 | 0.501 | gas |
| Butane | CH3(CH2)2CH3 | 138 | 275 | 0.579 | gas |
| Pentane | CH3(CH2)3CH3 | 143 | 309 | 0.626 | liquid |
| Hexane | CH3(CH2)4CH3 | 178 | 342 | 0.657 | liquid |
| Heptane | CH3(CH2)5CH3 | 182 | 372 | 0.684 | liquid |
| Octane | CH3(CH2)6CH3 | 216 | 399 | 0.703 | liquid |
| Nonane | CH3(CH2)7CH3 | 219 | 424 | 0.708 | liquid |
| Octane | CH3(CH2)8CH3 | 243 | 447 | 0.730 | liquid |
II.Chemical properties
(i)Burning.
Alkanes burn with a blue/non-luminous non-sooty/non-smoky flame in excess air to form carbon(IV) oxide and water.
Alkane + Air -> carbon(IV) oxide + water (excess air/oxygen)
Alkanes burn with a blue/non-luminous no-sooty/non-smoky flame in limited air to form carbon(II) oxide and water.
Alkane + Air -> carbon(II) oxide + water (limited air)
Examples
1.(a) Methane when ignited burns with a blue non sooty flame in excess air to form carbon(IV) oxide and water.
Methane + Air -> carbon(IV) oxide + water (excess air/oxygen)
CH4(g) + 2O2(g) -> CO2(g) + 2H2O(l/g)
(b) Methane when ignited burns with a blue non sooty flame in limited air to form carbon(II) oxide and water.
Methane + Air -> carbon(II) oxide + water (excess air/oxygen)
2CH4(g) + 3O2(g) -> 2CO(g) + 4H2O(l/g)
2.(a) Ethane when ignited burns with a blue non sooty flame in excess air to form carbon(IV) oxide and water.
Ethane + Air -> carbon(IV) oxide + water (excess air/oxygen)
2C2H6(g) + 7O2(g) -> 4CO2(g) + 6H2O(l/g)
(b) Ethane when ignited burns with a blue non sooty flame in limited air to form carbon(II) oxide and water.
Ethane + Air -> carbon(II) oxide + water (excess air/oxygen)
2C2H6(g) + 5O2(g) -> 4CO(g) + 6H2O(l/g)
3.(a) Propane when ignited burns with a blue non sooty flame in excess air to form carbon(IV) oxide and water.
Propane + Air -> carbon(IV) oxide + water (excess air/oxygen)
C3H8(g) + 5O2(g) -> 3CO2(g) + 4H2O(l/g)
(b) Ethane when ignited burns with a blue non sooty flame in limited air to form carbon(II) oxide and water.
Ethane + Air -> carbon(II) oxide + water (excess air/oxygen)
2C3H8(g) + 7O2(g) -> 6CO(g) + 8H2O(l/g)
ii)Substitution
Substitution reaction is one in which a hydrogen atom is replaced by a halogen in presence of ultraviolet light.
Alkanes react with halogens in presence of ultraviolet light to form halogenoalkanes.
During substitution:
(i)the halogen molecule is split into free atom/radicals.
(ii)one free halogen radical/atoms knock /remove one hydrogen from the alkane leaving an alkyl radical.
(iii) the alkyl radical combine with the other free halogen atom/radical to form halogenoalkane.
(iv)the chlorine atoms substitute repeatedly in the alkane. Each substitution removes a hydrogen atom from the alkane and form hydrogen halide.
(v)substitution stops when all the hydrogen in alkanes are replaced with halogens.
Substitution reaction is a highly explosive reaction in presence of sunlight / ultraviolet light that act as catalyst.
Examples of substitution reactions
Methane has no effect on bromine or chlorine in diffused light/dark. In sunlight , a mixture of chlorine and methane explode to form colourless mixture of chloromethane and hydrogen chloride gas. The pale green colour of chlorine gas fades.
Chemical equation
1.(a)Methane + chlorine -> Chloromethane + Hydrogen chloride
CH4(g) + Cl2(g) -> CH3Cl (g) + HCl (g)
H H
H C H + Cl Cl -> H C Cl + H Cl
H H
(b) Chloromethane + chlorine -> dichloromethane + Hydrogen chloride
CH3Cl (g) + Cl2(g) -> CH2Cl2 (g) + HCl (g)
H H
H C Cl + Cl Cl -> H C Cl + H Cl
H Cl
(c) dichloromethane + chlorine -> trichloromethane + Hydrogen chloride
CH2Cl2 (g) + Cl2(g) -> CHCl3 (g) + HCl (g)
Cl H
H C Cl + Cl Cl -> Cl C Cl + H Cl
H Cl
(c) trichloromethane + chlorine -> tetrachloromethane + Hydrogen chloride
CHCl3 (g) + Cl2(g) -> CCl4 (g) + HCl (g)
H Cl
Cl C Cl + Cl Cl -> Cl C Cl + H Cl
Cl Cl
Ethane has no effect on bromine or chlorine in diffused light/dark. In sunlight , a mixture of bromine and ethane explode to form colourless mixture of bromoethane and hydrogen chloride gas. The red/brown colour of bromine gas fades.
Chemical equation
(a)Ethane + chlorine -> Chloroethane + Hydrogen chloride
CH3CH3(g) + Br2(g) -> CH3CH2Br (g) + HBr (g)
H H H H
H C C H + Br Br -> H C C H + H Br
H H H Br
Bromoethane
H H H Br
H C C H + Br Br -> H C C H + H Br
H Br H Br
1,1-dibromoethane
H Br H Br
H C C H + Br Br -> H C C Br + H Br
H Br H Br
1,1,1-tribromoethane
H Br H Br
H C C Br + Br Br -> H C C Br + H Br
H Br Br Br
1,1,1,2-tetrabromoethane
H Br H Br
H C C Br + Br Br -> Br C C Br + H Br
Br Br Br Br
1,1,1,2,2-pentabromoethane
H Br Br Br
Br C C Br + Br Br -> Br C C Br + H Br
Br Br Br Br
1,1,1,2,2,2-hexabromoethane
Uses of alkanes
1.Most alkanes are used as fuel e.g. Methane is used as biogas in homes. Butane is used as the Laboratory gas.
2.On cracking ,alkanes are a major source of Hydrogen for the manufacture of ammonia/Haber process.
3.In manufacture of Carbon black which is a component in printers ink.
4.In manufacture of useful industrial chemicals like methanol, methanol, and chloromethane.
