Practice questions Organic chemistry
1. A student mixed equal volumes of Ethanol and butanoic acid. He added a few drops of concentrated Sulphuric (VI) acid and warmed the mixture
(i) Name and write the formula of the main products Name………………………………….
Formula……………………………………..
(ii) Which homologous series does the product named in (i) above belong?
2. The structure of the monomer phenyl ethene is given below:-
a) Give the structure of the polymer formed when four of the monomers are added together
b) Give the name of the polymer formed in (a) above
3. Explain the environmental effects of burning plastics in air as a disposal method 4. Write chemical equation to represent the effect of heat on ammonium carbonate
5. Sodium octadecanoate has a chemical formula CH3(CH2)6 COO–Na+, which is used as soap.
Explain why a lot of soap is needed when washing with hard water
6. A natural polymer is made up of the monomer:
(a) Write the structural formula of the repeat unit of the polymer (b) When 5.0 x 10-5 moles of the polymer were hydrolysed, 0.515g of the monomer were obtained.
Determine the number of the monomer molecules in this polymer.
(C = 12; H = 1; N = 14; O =16)
7. The formula below represents active ingredients of two cleansing agents A and B
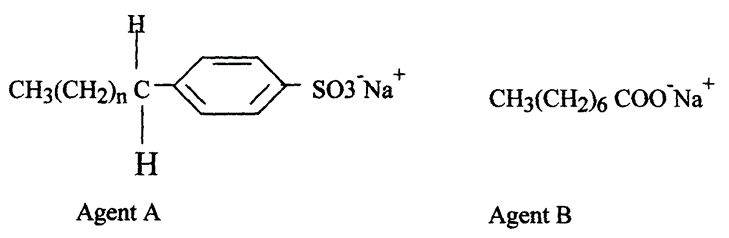
Which one of the cleansing agents would be suitable to be used in water containing magnesium hydrogen carbonate? Explain
Ethanol and Pentane are miscible liquids. Explain how water can be used to separate a mixture of ethanol and pentane
10.
(a) What is absolute ethanol?
| (b) State two conditions required for process G to take place efficiently 11. (a) (i) The table below shows the volume of oxygen obtained per unit time when hydrogen peroxide was decomposed in the presence of manganese (IV) Oxide. Use it to answer the questions that follow:- Time in seconds | Volume of Oxygen evolved (cm3) |
| 0 30 60 90 120 150 180 210 240 270 300 | 0 10 19 27 34 38 43 45 45 45 45 |
(i) Plot a graph of volume of oxygen gas against time (ii) Determine the rate of reaction at time 156 seconds (iii) From the graph, find the time taken for 18cm3 of oxygen to be produced (iv) Write a chemical equation to show how hydrogen peroxide decomposes in the presence of manganese (IV) Oxide
(b) The diagram below shows how a Le’clanche (Dry cell) appears:-
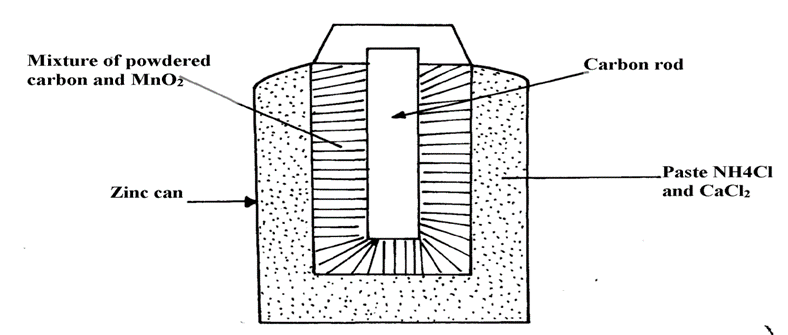
(i) What is the function of MnO2 in the cell above?
(ii) Write the equation of a reaction that occurs at the cathode
(iii) Calculate the mass of Zinc that is consumed when a current of 0.1amperes flows through the above cell for 30minutes (1F =96500c Zn =65)
11. (a) Give the IUPAC names of the following compounds:
(i) CH3COOCH2CH3 *
(ii)
12. a) State two factors that affect the properties of a polymer
b) Name the compound with the formula below :
CH3CH2CH2ONa
c. Explain how compounds CH3CH2COOH and CH3CH2CH2OH can be distinguished chemically
14.A hydrated salt has the following composition by mass. Iron 20.2 %, oxygen 23.0%, sulphur 11.5%, water 45.3%
i) Determine the formula of the hydrated salt (Fe=56, S=32, O=16, H=11) ii) 6.95g of the hydrated salt in c(i) above were dissolved in distilled water and the total volume made to 250cm3 of solution. Calculate the concentration of the resulting salt solution in moles per litre. (Given that the molecula mass of the salt is 278)
13. Write an equation to show products formed for the complete combustion of CH = CH
iii) Explain one disadvantage of continued use of items made from the compound formed in step III
14. Give the IUPAC name for each of the following organic compounds;
i) CH3 – CH – CH2 – CH3
OH
ii)CH3 – CH – CH2 – CH2 – CH3
C2H5
iii)CH3COOCH2CH2CH3
15. The structure below represents a cleansing agent.
O
R – S – O–Na+
O
a) State the type of cleansing agent represented above b) State one advantage and one disadvantage of using the above cleansing agent.
16. The structure below shows part of polymer .Use it to answer the questions that follow.
CH3 CH3 CH3
ï ï ï
― CH – CH2 – CH- CH2 – CH – CH2 ―
a) Derive the structure of the monomer
b) Name the type of polymerization represented above
17. a) Write an equation showing how ammonium nitrate may be prepared starting with ammonia gas
(b) Calculate the maximum mass of ammonium nitrate that can be prepared using 5.3kg of ammonia (H=1, N=14, O=16)
18. (a) What is meant by the term, esterification?
(b) Draw the structural formulae of two compounds that may be reacted to form ethylpropanoate
19. (a) Draw the structure of pentanoic acid
(b) Draw the structure and give the name of the organic compound formed when ethanol reacts with pentanoic acid in presence of concentrated sulphuric acid
20. Substances A and B are represented by the formulae ROH and RCOOH respectively.
They belong to two different homologous series of organic compounds. If both A and B
react with potassium metal:
(a) Name the common product produced by both (b) State the observation made when each of the samples A and B are reacted with sodium hydrogen carbonate
(i) A
(ii) B
21. Below are structures of particles. Use it to answer questions that follow. In each case only electrons in the outermost energy level are shown
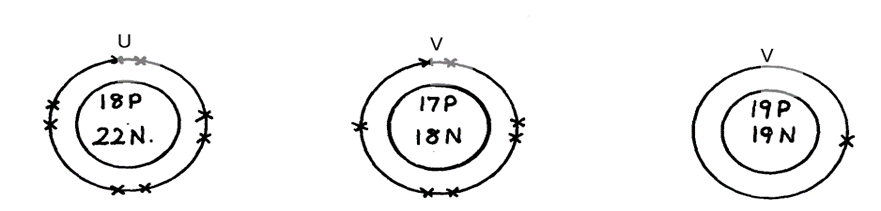
key
P = Proton
N = Neutron
X = Electron
(a) Identify the particle which is an anion
22. Plastics and rubber are extensively used to cover electrical wires.
(a) What term is used to describe plastic and rubbers used in this way?
(b) Explain why plastics and rubbers are used this way
23. Y grams of a radioactive isotope take 120days to decay to 3.5grams. The half-life period of the isotope is 20days
(a) Find the initial mass of the isotope
(b) Give one application of radioactivity in agriculture
24. RCOO–Na+ and RCH2OSO3–Na+ are two types of cleansing agents;
i) Name the class of cleansing agents to which each belongs
ii) Which one of these agents in (i) above would be more suitable when washing with water from the Indian ocean. Explain iii) Both sulphur (IV) oxide and chlorine are used bleaching agents. Explain the difference in their bleaching properties
