(a) Occurrence of Hydrogen Sulphide
Hydrogen sulphide is found in volcanic areas as a gas or dissolved in water from geysers and hot springs in active volcanic areas of the world e.g. Olkaria and Hells gate near Naivasha in Kenya.
It is present in rotten eggs and human excreta.
(b) Preparation
Hydrogen sulphide is prepared in a school laboratory by heating Iron (II) sulphide with dilute hydrochloric acid.
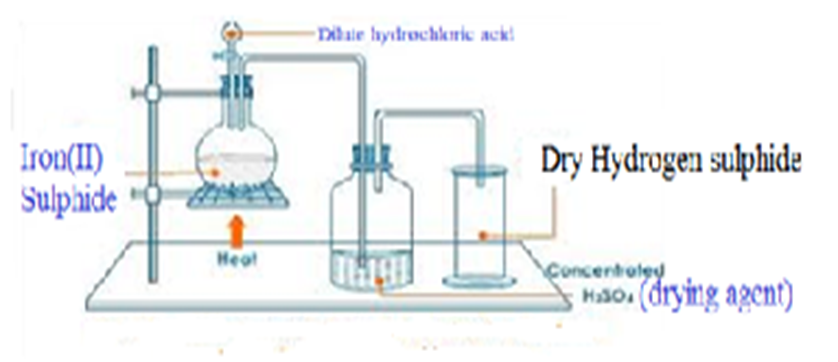
Practical Questions on Hydrogen Sulphide
(c) Properties of Hydrogen sulphide(Questions)
1. Write the equation for the reaction for the school laboratory preparation of Hydrogen sulphide.
Chemical equation: FeS (s) + 2HCl (aq) -> H2S (g) FeCl2 (aq)
2. State three physical properties unique to Hydrogen sulphide.
Hydrogen sulphide is a colourless gas with characteristic pungent poisonous smell of rotten eggs. It is soluble in cold water but insoluble in warm water. It is denser than water and turns blue litmus paper red.
3. Hydrogen sulphide exist as a dibasic acid when dissolved in water. Using a chemical equation show how it ionizes in aqueous state.
H2S(aq) -> H+(aq) + HS–(aq)
H2S(aq) -> 2H+(aq) + S2- (aq)
Hydrogen sulphide therefore can form both normal and acid salts e.g
Sodium hydrogen sulphide and sodium sulphide both exist
4. State and explain one gaseous impurity likely to be present in the gas jar containing hydrogen sulphide above.
Hydrogen/ H2
Iron(II)sulphide contains Iron as impurity .The iron will react with dilute hydrochloric acid to form iron(II)chloride and produce hydrogen gas that mixes with hydrogen sulphide gas.
5. State and explain the observations made when a filter paper dipped in Lead(II) ethanoate /Lead (II) nitrate(V) solution is put in a gas jar containing hydrogen sulphide gas.
Observations
Moist Lead(II) ethanoate /Lead (II) nitrate(V) paper turns black.
Explanation
When hydrogen sulphide is bubbled in a metallic salt solution, a metallic sulphide is formed.
All sulphides are insoluble black salts except sodium sulphide, potassium sulphide and ammonium sulphides.
Hydrogen sulphide gas blackens moist Lead (II) ethanoate /Lead (II) nitrate(V) paper .
The gas reacts with Pb2+ in the paper to form black Lead(II)sulphide.
This is the chemical test for the presence of H2S other than the physical smell of rotten eggs.
Chemical equations
Pb2+(aq) + H2S -> PbS + 2H+(aq)
(black)
Fe2+(aq) + H2S -> FeS + 2H+(aq)
(black)
Zn2+(aq) + H2S -> ZnS + 2H+(aq)
(black)
Cu2+(aq) + H2S -> CuS + 2H+(aq)
(black)
2Cu+(aq) + H2S -> Cu2S + 2H+(aq)
(black)
6. Dry hydrogen sulphide was ignited as below.
| Dry Hydrogen sulphide gas |
| Flame A |
(i) State the observations made in flame A
Hydrogen sulphide burns in excess air with a blue flame to form sulphur(IV)oxide gas and water.
Chemical equation: 2H2S(g) + 3O2(g) -> 2H2O(l) + 2SO2(g)
Hydrogen sulphide burns in limited air with a blue flame to form sulphur solid and water.
Chemical equation: 2H2S(g) + O2(g) -> 2H2O(l) + 2S(s)
7. Hydrogen sulphide is a strong reducing agent that is oxidized to yellow solid sulphur as precipitate. The following experiments illustrate the reducing properties of Hydrogen sulphide.
(a)Turns Orange acidified potassium dichromate(VI) to green
Experiment:
(i)Pass a stream of Hydrogen sulphide gas in a test tube containingacidified potassium dichromate (VI) solution. or;
(ii)Dip a filter paper soaked in acidified potassium dichromate (VI) into a gas jar containing Hydrogen sulphide gas.
Observation:
Orange acidified potassium dichromate (VI) turns to green.
Yellow solid residue.
Explanation:
Hydrogen sulphide gas reduces acidified potassium dichromate(VI) from orange Cr2O72- ions to green Cr3+ ions leaving a yellow solid residue as itself is oxidized to sulphur.
Chemical/ionic equation:
4H2S(aq) + Cr2O72-(aq) +6H+(aq) -> 4S(aq) + 2Cr3+(aq) + 7H2O(l)
This test is used for differentiating Hydrogen sulphide and sulphur (IV)oxide gas.
Sulphur(IV)oxide also reduces acidified potassium dichromate(VI) from orange Cr2O72- ions to green Cr3+ ions without leaving a yellow residue.
(b)Decolorizes acidified potassium manganate(VII)
Experiment:
(i)Pass a stream of Sulphur(IV) oxide gas in a test tube containingacidified potassium manganate(VII) solution. or;
(ii)Dip a filter paper soaked in acidified potassium manganate(VII) into a gas jar containing Hydrogen Sulphide gas.
Observation:
Purple acidified potassium manganate(VII) turns to colourless/ acidified potassium manganate(VII) is decolorized.
Yellow solid residue.
Explanation:
Hydrogen sulphide gas reduces acidified potassium manganate(VII) from purple MnO4– ions to green Mn2+ ions leaving a residue as the gas itself is oxidized to sulphur.
Chemical/ionic equation:
5H2S(g) + 2MnO4– (aq) +6H+(aq) -> 5S (s) + 2Mn2+(aq) + 8H2O(l)
(purple) (colourless)
This is another test for differentiating Hydrogen sulphide and Sulphur(IV) oxide gas.
Sulphur(IV) oxide also decolorizes acidified potassium manganate(VII) from purple MnO4– ions to colourless Mn2+ ions leaving no yellow residue.
(c)Decolorizes bromine water
Experiment:
(i)Pass a stream of Hydrogen sulphide gas in a test tube containingbromine water . or;
(ii)Put three drops of bromine water into a gas jar containing Hydrogen sulphide gas. Swirl.
Observation:
Yellow bromine water turns to colourless/ bromine water is decolorized.
Yellow solid residue
Explanation:
Hydrogen sulphide gas reduces yellow bromine water to colourless hydrobromic acid (HBr) leaving a yellow residue as the gas itself is oxidized to sulphur.
Chemical/ionic equation:
H2 S(g) + Br2 (aq) -> S (s) + 2HBr(aq)
(yellow solution) (yellow solid) (colourless)
This is another test for differentiating Hydrogen sulphide and Sulphur(IV) oxide gas.
Sulphur(IV) oxide also decolorizes acidified potassium manganate(VII) from purple MnO4– ions to colourless Mn2+ ions leaving no yellow residue.
(d)Reduces Iron(III) Fe3+ salts to Iron(II) salts Fe2+
Experiment:
(i)Pass a stream of Hydrogen sulphide gas in a test tube containingabout 3 cm3 of Iron (III)chloride solution. or;
(ii)Place about 3cm3 of Iron (III)chloride solution into a gas jar containing Hydrogen sulphide gas. Swirl.
Observation:
Yellow/brown Iron (III)chloride solution turns to green.
Yellow solid
Explanation:
Hydrogen sulphide gas reduces Iron (III)chloride solution from yellow/brown Fe3+ ions to green Fe2+ ions leaving a yellow residue.The gas is itself oxidized to sulphur.
Chemical/ionic equation:
H2S(aq) + 2Fe3+ (aq) -> S (s) + Fe2+(aq) + 2H+(aq)
(yellow solution) (yellow residue) (green)
(e)Reduces Nitric(V)acid to Nitrogen(IV)oxide gas
Experiment:
(i)Pass a stream of Hydrogen sulphide gas in a test tube containingabout 3 cm3 of concentrated nitric(V)acid. or;
(ii)Place about 3cm3 of concentrated nitric(V)acid into a gas jar containing Hydrogen sulphide gas. Swirl.
Observation:
Brown fumes of a gas evolved/produced.
Yellow solid residue
Explanation:
Hydrogen sulphide gas reduces concentrated nitric(V)acid to brown nitrogen(IV)oxide gas itself oxidized to yellow sulphur.
Chemical/ionic equation:
H2S(g) + 2HNO3 (l) -> 2H2O(l) + S (s) + 2NO2 (g)
(yellow residue) (brown fumes)
(f)Reduces sulphuric(VI)acid to Sulphur
Experiment:
(i)Pass a stream of Hydrogen sulphide gas in a test tube containingabout 3 cm3 of concentrated sulphuric(VI)acid. or;
(ii)Place about 3cm3 of concentrated sulphuric (VI) acid into a gas jar containing Hydrogen sulphide gas. Swirl.
Observation:
Yellow solid residue
Explanation:
Hydrogen sulphide gas reduces concentrated sulphuric(VI)acid to yellow sulphur.
Chemical/ionic equation:
3H2S(g) + H2SO4 (l) -> 4H2O(l) + 4S (s)
(yellow residue)
(g)Reduces Hydrogen peroxide to water
Experiment:
(i)Pass a stream of Hydrogen sulphide gas in a test tube containingabout 3 cm3 of 20 volume hydrogen peroxide.
Observation:
Yellow solid residue
Explanation:
Hydrogen sulphide gas reduces 20 volume hydrogen peroxide to water and itself oxidized to yellow sulphur
Chemical/ionic equation:
H2S(g) + H2O2 (l) -> 2H2O(l) + S (s)
(yellow residue)
8.Name the salt formed when:
(i)equal volumes of equimolar hydrogen sulphide neutralizes sodium hydroxide solution:
Sodium hydrogen sulphide
Chemical/ionic equation:
H2S(g) + NaOH (l) -> H2O(l) + NaHS (aq)
(ii) hydrogen sulphide neutralizes excess concentrated sodium hydroxide solution:
Sodium sulphide
Chemical/ionic equation:
H2S(g) + 2NaOH (l) -> 2H2O(l) + Na2S (aq)
Practice
Hydrogen sulphide gas was bubbled into a solution of metallic nitrate(V)salts as in the flow chart below
| Hydrogen sulphide |
| Blue solution |
| Black solid |
| Green solution |
| Brown solution |
(a)Name the black solid Copper(II)sulphide
(b)Identify the cation responsible for the formation of:
I. Blue solution Cu2+(aq)
II. Green solution Fe2+(aq)
III. Brown solution Fe3+(aq)
(c)Using acidified potassium dichromate(VI) describe how you would differentiate between sulphur(IV)Oxide and hydrogen sulphide
-Bubble the gases in separate test tubes containing acidified Potassium dichromate(VI) solution.
-Both changes the Orange colour of acidified Potassium dichromate(VI) solution to green.
-Yellow solid residue/deposit is formed with Hydrogen sulphide
Chemical/ionic equation:
4H2S(aq) + Cr2O72-(aq) +6H+(aq) -> 4S(aq) + 2Cr3+(aq) + 7H2O(l)
3SO32-(aq) + Cr2O72-(aq) +8H+(aq) -> 3SO42-(aq) + 2Cr3+(aq) + 4H2O(l)
(d)State and explain the observations made if a burning splint is introduced at the mouth of a hydrogen sulphide generator.
Observation
Gas continues burning with a blue flame
Explanation: Hydrogen sulphide burns in excess air with a blue flame to form sulphur(IV)oxide gas and water.
Chemical equation: 2H2S(g)+ 3O2(g) -> 2H2O(l) + 2SO2 (g)
(v)Sulphate (VI) (SO42-)and Sulphate(IV) (SO32-) salts
1. Sulphate (VI) (SO42-) salts are normal and acid salts derived from Sulphuric (VI)acid H2SO4.
2. Sulphate(IV) (SO32-) salts are normal and acid salts derived from Sulphuric (IV)acid H2SO3.
3. Sulphuric (VI)acid H2SO4 is formed when sulphur(VI)oxide gas is bubbled in water.
The acid exist as a dibasic acid with two ionisable hydrogen. It forms therefore the Sulphate (VI) (SO42-) and hydrogen sulphate (VI) (HSO4–) salts.
i.e.
H2SO4 (aq) -> 2H+(aq) + SO42-(aq)
H2SO4 (aq) -> H+(aq) + HSO4 –(aq)
All Sulphate (VI) (SO42-) salts dissolve in water/are soluble except Calcium (II) sulphate (VI) (CaSO4), Barium (II) sulphate (VI) (BaSO4) and Lead (II) sulphate (VI) (PbSO4)
All Hydrogen sulphate (VI) (HSO3–) salts exist in solution/dissolved in water. Sodium (I) hydrogen sulphate (VI) (NaHSO4), Potassium (I) hydrogen sulphate (VI) (KHSO4) and Ammonium hydrogen sulphate (VI) (NH4HSO4) exist also as solids.
Other Hydrogen sulphate (VI) (HSO4–) salts do not exist except those of Calcium (II) hydrogen sulphate (VI) (Ca (HSO4)2) and Magnesium (II) hydrogen sulphate (VI) (Mg (HSO4)2).
4. Sulphuric (IV)acid H2SO3 is formed when sulphur(IV)oxide gas is bubbled in water.
The acid exist as a dibasic acid with two ionisable hydrogen. It forms therefore the Sulphate (IV) (SO32-) and hydrogen sulphate (VI) (HSO4–) salts.
i.e.
H2SO3 (aq) -> 2H+(aq) + SO32-(aq)
H2SO3 (aq) -> H+(aq) + HSO3 –(aq)
All Sulphate (IV) (SO32-) salts dissolve in water/are soluble except Calcium (II) sulphate (IV) (CaSO3), Barium (II) sulphate (IV) (BaSO3) and Lead (II) sulphate (IV) (PbSO3)
All Hydrogen sulphate (IV) (HSO3–) salts exist in solution/dissolved in water. Sodium (I) hydrogen sulphate (IV) (NaHSO3), Potassium (I) hydrogen sulphate (IV) (KHSO3) and Ammonium hydrogen sulphate (IV) (NH4HSO3) exist also as solids.
Other Hydrogen sulphate (IV) (HSO3–) salts do not exist except those of Calcium (II) hydrogen sulphate (IV) (Ca (HSO3)2) and Magnesium (II) hydrogen sulphate (IV) (Mg (HSO3)2).
5.The following experiments show the effect of heat on sulphate(VI) (SO42-)and sulphate(IV) (SO32-) salts:
Experiment:
In a clean dry test tube place separately about 1.0g of :
Zinc(II)sulphate (VI), Iron(II)sulphate(VI), Copper(II)sulphate(VI),Sodium (I) sulphate (VI), Sodium (I) sulphate (IV).Heat gently then strongly. Test any gases produced using litmus papers.
Observations:
-Colourless droplets of liquid forms on the cooler parts of the test tube in all cases.
-White solid residue is left in case of Zinc (II)sulphate(VI),Sodium (I) sulphate (VI) and Sodium (I) sulphate (IV).
-Colour changes from green to brown /yellow in case of Iron (II)sulphate(VI)
-Colour changes from blue to white then black in case of Copper (II) sulphate (VI)
-Blue litmus paper remain and blue and red litmus paper remain red in case of Zinc(II)sulphate(VI), Sodium (I) sulphate (VI) and Sodium (I) sulphate (IV)
-Blue litmus paper turns red and red litmus paper remain red in case of Iron (II)sulphate(VI) and Copper (II) sulphate (VI).
Explanation
(i)All Sulphate (VI) (SO42-) salts exist as hydrated salts with water of crystallization that condenses and collects on cooler parts of test tube as a colourless liquid on gentle heating. e.g.
K2SO4.10H2O(s) -> K2SO4(s) + 10H2O(l)
Na2SO4.10H2O(s) -> Na2SO4(s) + 10H2O(l)
MgSO4.7H2O(s) -> MgSO4(s) + 7H2O(l)
CaSO4.7H2O(s) -> CaSO4(s) + 7H2O(l)
ZnSO4.7H2O(s) -> ZnSO4(s) + 7H2O(l)
FeSO4.7H2O(s) -> FeSO4(s) + 7H2O(l)
Al2(SO4)3.6H2O(s) -> Al2(SO4)3 (s) + 6H2O(l)
CuSO4.5H2O(s) -> CuSO4(s) + 5H2O(l)
All Sulphate (VI) (SO42-) salts do not decompose on heating except Iron (II) sulphate (VI) and Copper (II) sulphate (VI).
(i)Iron (II) sulphate (VI) decomposes on strong heating to produce acidic sulphur (IV)oxide and sulphur(VI)oxide gases. Iron(III)oxide is formed as a brown /yellow residue.
Chemical equation
2FeSO4 (s) -> Fe2O3(s) + SO2(g) + SO3(g)
This reaction is used for the school laboratory preparation of small amount of sulphur(VI)oxide gas.
Sulphur (VI) oxide readily /easily solidifies as white silky needles when the mixture is passed through freezing mixture/ice cold water.
Sulphur (IV) oxide does not.
(ii) Copper(II)sulphate(VI) decomposes on strong heating to black copper (II) oxide and Sulphur (VI) oxide gas.
Chemical equation
2CuSO4 (s) -> CuO(s) + SO3(g)
This reaction is used for the school laboratory preparation of small amount of sulphur(VI)oxide gas.
6. The following experiments show the test for the presence of sulphate (VI) (SO42-)and sulphate(IV) (SO32-) ions in a sample of a salt/compound:
Experiments/Observations:
(a)Using Lead(II)nitrate(V)
I. To about 5cm3 of a salt solution in a test tube add four drops of Lead(II)nitrate(V)solution. Preserve.
| Observation | Inference |
| White precipitate/ppt | SO42- , SO32- , CO32- , Cl– ions |
II. To the preserved sample in (I) above, add six drops of 2M nitric(V) acid . Preserve.
Observation 1
| Observation | Inference |
| White precipitate/ppt persists | SO42- , Cl– ions |
Observation 2
| Observation | Inference |
| White precipitate/ppt dissolves | SO32- , CO32- , ions |
III.(a)To the preserved sample observation 1 in (II) above, Heat to boil.
Observation 1
| Observation | Inference |
| White precipitate/ppt persists on boiling | SO42- ions |
Observation 2
| Observation | Inference |
| White precipitate/ppt dissolves on boiling | Cl – ions |
.(b)To the preserved sample observation 2 in (II) above, add 4 drops of acidified potassium manganate(VII) /dichromate(VI).
Observation 1
| Observation | Inference |
| (i)acidified potassium manganate(VII)decolorized (ii)Orange colour of acidified potassium dichromate(VI) turns to green | SO32- ions |
Observation 2
| Observation | Inference |
| (i)acidified potassium manganate(VII) not decolorized (ii)Orange colour of acidified potassium dichromate(VI) does not turns to green | CO32- ions |
Experiments/Observations:
(b)Using Barium(II)nitrate(V)/ Barium(II)chloride
I. To about 5cm3 of a salt solution in a test tube add four drops of Barium(II) nitrate (V) / Barium(II)chloride. Preserve.
| Observation | Inference |
| White precipitate/ppt | SO42- , SO32- , CO32- ions |
II. To the preserved sample in (I) above, add six drops of 2M nitric(V) acid . Preserve.
Observation 1
| Observation | Inference |
| White precipitate/ppt persists | SO42- , ions |
Observation 2
| Observation | Inference |
| White precipitate/ppt dissolves | SO32- , CO32- , ions |
III.To the preserved sample observation 2 in (II) above, add 4 drops of acidified potassium manganate(VII) /dichromate(VI).
Observation 1
| Observation | Inference |
| (i)acidified potassium manganate(VII)decolorized (ii)Orange colour of acidified potassium dichromate(VI) turns to green | SO32- ions |
Observation 2
| Observation | Inference |
| (i)acidified potassium manganate(VII) not decolorized (ii)Orange colour of acidified potassium dichromate(VI) does not turns to green | CO32- ions |
Explanations
Using Lead(II)nitrate(V)
(i)Lead(II)nitrate(V) solution reacts with chlorides(Cl–), Sulphate (VI) salts (SO42- ), Sulphate (IV)salts (SO32-) and carbonates(CO32-) to form the insoluble white precipitate of Lead(II)chloride, Lead(II)sulphate(VI), Lead(II) sulphate (IV) and Lead(II)carbonate(IV).
Chemical/ionic equation:
Pb2+(aq) + Cl– (aq) -> PbCl2(s)
Pb2+(aq) + SO42+ (aq) -> PbSO4 (s)
Pb2+(aq) + SO32+ (aq) -> PbSO3 (s)
Pb2+(aq) + CO32+ (aq) -> PbCO3 (s)
(ii)When the insoluble precipitates are acidified with nitric(V) acid,
– Lead(II)chloride and Lead(II)sulphate(VI) do not react with the acid and thus their white precipitates remain/ persists.
– Lead(II) sulphate (IV) and Lead(II)carbonate(IV) reacts with the acid to form soluble Lead(II) nitrate (V) and produce/effervesces/fizzes/bubbles out sulphur(IV)oxide and carbon(IV)oxide gases respectively.
. Chemical/ionic equation:
PbSO3 (s) + 2H+(aq) -> H2 O (l) + Pb2+(aq) + SO2 (g)
PbCO3 (s) + 2H+(aq) -> H2 O (l) + Pb2+(aq) + CO2 (g)
(iii)When Lead(II)chloride and Lead(II)sulphate(VI) are heated/warmed;
– Lead(II)chloride dissolves in hot water/on boiling(recrystallizes on cooling)
– Lead(II)sulphate(VI) do not dissolve in hot water thus its white precipitate persists/remains on heating/boiling.
(iv)When sulphur(IV)oxide and carbon(IV)oxide gases are produced;
– sulphur(IV)oxide will decolorize acidified potassium manganate(VII) and / or Orange colour of acidified potassium dichromate(VI) will turns to green. Carbon(IV)oxide will not.
Chemical equation:
5SO32-(aq) + 2MnO4– (aq) +6H+(aq) -> 5SO42-(aq) + 2Mn2+(aq) + 3H2O(l)
(purple) (colourless)
3SO32-(aq) + Cr2O72-(aq) +8H+(aq) -> 3SO42-(aq) + 2Cr3+(aq) + 4H2O(l)
(Orange) (green)
– Carbon(IV)oxide forms an insoluble white precipitate of calcium carbonate if three drops of lime water are added into the reaction test tube when effervescence is taking place. Sulphur(IV)oxide will not.
Chemical equation:
Ca(OH)2(aq) + CO2 (g) -> CaCO3(s) + H2O(l)
These tests should be done immediately after acidifying to ensure the gases produced react with the oxidizing agents/lime water.
Using Barium(II)nitrate(V)/ Barium(II)Chloride
(i)Barium(II)nitrate(V) and/ or Barium(II)chloride solution reacts with Sulphate (VI) salts (SO42- ), Sulphate (IV)salts (SO32-) and carbonates(CO32-) to form the insoluble white precipitate of Barium(II)sulphate(VI), Barium(II) sulphate (IV) and Barium(II)carbonate(IV).
Chemical/ionic equation:
Ba2+(aq) + SO42+ (aq) -> BaSO4 (s)
Ba2+(aq) + SO32+ (aq) -> BaSO3 (s)
Ba2+(aq) + CO32+ (aq) -> BaCO3 (s)
(ii)When the insoluble precipitates are acidified with nitric(V) acid,
– Barium (II)sulphate(VI) do not react with the acid and thus its white precipitates remain/ persists.
– Barium(II) sulphate (IV) and Barium(II)carbonate(IV) reacts with the acid to form soluble Barium(II) nitrate (V) and produce /effervesces /fizzes/ bubbles out sulphur(IV)oxide and carbon(IV)oxide gases respectively.
. Chemical/ionic equation:
BaSO3 (s) + 2H+(aq) -> H2 O (l) + Ba2+(aq) + SO2 (g)
BaCO3 (s) + 2H+(aq) -> H2 O (l) + Ba2+(aq) + CO2 (g)
(iii) When sulphur(IV)oxide and carbon(IV)oxide gases are produced;
– sulphur(IV)oxide will decolorize acidified potassium manganate(VII) and / or Orange colour of acidified potassium dichromate(VI) will turns to green. Carbon(IV)oxide will not.
Chemical equation:
5SO32-(aq) + 2MnO4– (aq) +6H+(aq) -> 5SO42-(aq) + 2Mn2+(aq) + 3H2O(l)
(purple) (colourless)
3SO32-(aq) + Cr2O72-(aq) +8H+(aq) -> 3SO42-(aq) + 2Cr3+(aq) + 4H2O(l)
(Orange) (green)
– Carbon(IV)oxide forms an insoluble white precipitate of calcium carbonate if three drops of lime water are added into the reaction test tube when effervescence is taking place. Sulphur(IV)oxide will not.
Chemical equation:
Ca(OH)2(aq) + CO2 (g) -> CaCO3(s) + H2O(l)
These tests should be done immediately after acidifying to ensure the gases produced react with the oxidizing agents/lime water.
2. Study the flow chart below and answer the questions that follow.
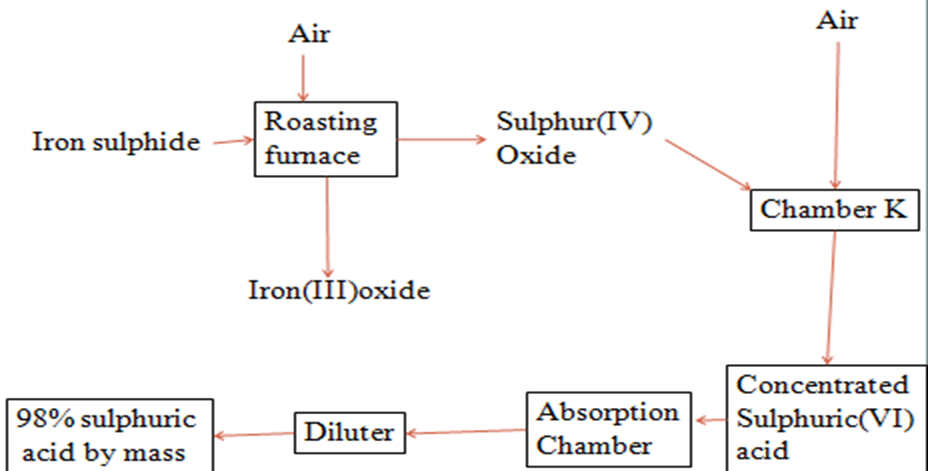
(i)Write equation for the reaction taking place at:
I.The roasting furnace (1mk)
2FeS2 (s) + 5O2 (g) -> 2FeO(s) + 4SO2 (g)
II.The absorption tower (1mk)
H2SO4 (l) + SO3 (g) -> H2S2O7(l)
III.The diluter (1mk)
H2S2O7(l) + H2 O(l) -> 2H2SO4 (l)
(ii)The reaction taking place in chamber K is
SO2 (g) + 1/2O2 (g) SO3 (g)
I. Explain why it is necessary to use excess air in chamber K
To ensure all the SO2 reacts
II.Name another substance used in chamber K
Vanadium(V)oxide
3.(a)Describe a chemical test that can be used to differentiate between sodium sulphate (IV) and sodium sulphate (VI).
Add acidified Barium nitrate(V)/chloride.
White precipitate formed with sodium sulphate (VI)
No white precipitate formed with sodium sulphate (IV)
(b)Calculate the volume of sulphur (IV) oxide formed when 120 kg of copper is reacted with excess concentrated sulphuric(VI)acid.(Cu = 63.5 ,1 mole of a gas at s.t.p =22.4dm3)
Chemical equation
Cu(s) + 2H2SO4(l) -> CuSO4(aq)+ H2O(l) + SO2 (g)
Mole ratio Cu(s: SO2 (g) = 1:1
Method 1
1 Mole Cu =63.5 g -> 1 mole SO2 = 22.4dm3
(120 x 1000) g -> (120 x 1000) g x 22.4.dm3)
63.5 g
= 42330.7087
Method 2
Moles of Cu = ( 120 x 1000 ) g =1889.7639 moles
63.5
Moles SO2 = Moles of Cu = 1889.7639 moles
Volume of SO2 = Mole x molar gas volume = (1889.7639 moles x 22.4)
= 42330.7114
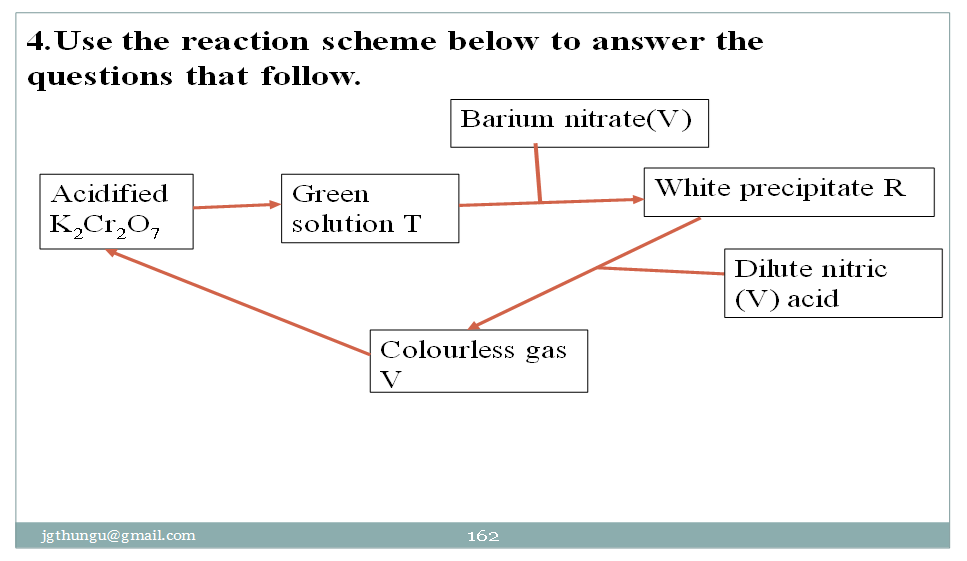
(a)Identify the:
(i)cation responsible for the green solution T
Cr3+
(ii)possible anions present in white precipitate R
CO32-, SO32-, SO42-
(b)Name gas V
Sulphur (IV)oxide
(c)Write a possible ionic equation for the formation of white precipitate R.
Ba2+ (aq) + CO32- (aq) -> BaCO3(s)
Ba2+ (aq) + SO32- (aq) -> BaSO3(s)
Ba2+ (aq) + SO42- (aq) -> BaSO4(s)
