B.ALKANOLS (Alcohols)
Alkanols belong to a homologous series of organic compounds with a general formula CnH2n +1 OH and thus -OH as the functional group .The 1st ten alkanols include
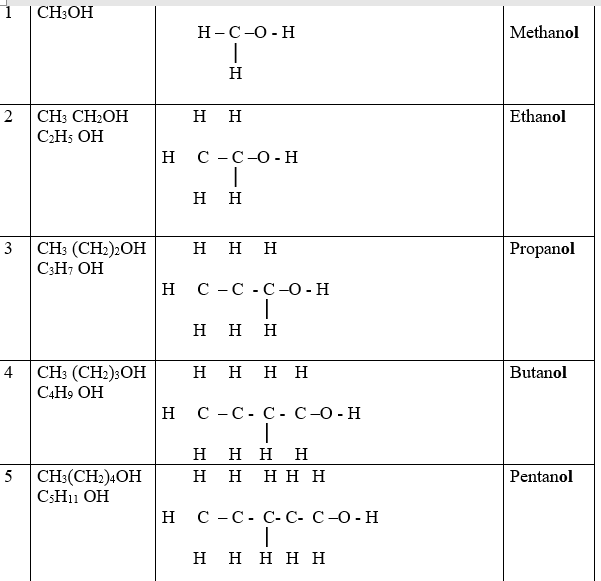
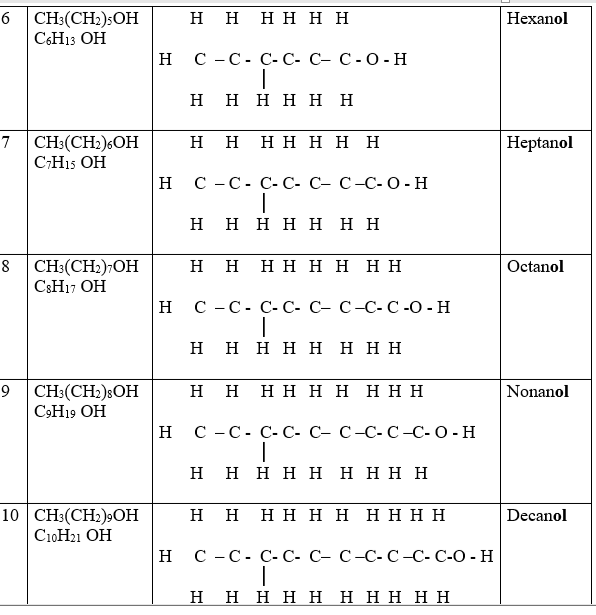
Alkanols like Hydrocarbons( alkanes/alkenes/alkynes) form a homologous series where:
(i)general name is derived from the alkane name then ending with “-ol”
(ii)the members have –OH as the fuctional group
(iii)they have the same general formula represented by R-OH where R is an alkyl group.
(iv) each member differ by –CH2 group from the next/previous.
(v)they show a similar and gradual change in their physical properties e.g. boiling and melting points.
(vi)they show similar and gradual change in their chemical properties.
B. ISOMERS OF ALKANOLS.
Alkanols exhibit both structural and position isomerism. The isomers are named by using the following basic guidelines:
(i)Like alkanes , identify the longest carbon chain to be the parent name.
(ii)Identify the position of the -OH functional group to give it the smallest /lowest position.
(iii) Identify the type and position of the side branches.
Practice examples of isomers of alkanols
(i)Isomers of propanol C3H7OH
CH3CH2CH2OH – Propan-1-ol
OH
CH3CHCH3 – Propan-2-ol
Propan-2-ol and Propan-1-ol are position isomers because only the position of the –OH functional group changes.
(ii)Isomers of Butanol C4H9OH
CH3 CH2 CH3 CH2 OH Butan-1-ol
CH3 CH2 CH CH3
OH Butan-2-ol
CH3
CH3 CH3 CH3
OH 2-methylpropan-2-ol
Butan-2-ol and Butan-1-ol are position isomers because only the position of the -OH functional group changes.
2-methylpropan-2-ol is both a structural and position isomers because both the position of the functional group and the arrangement of the atoms in the molecule changes.
(iii)Isomers of Pentanol C5H11OH
CH3 CH2 CH2CH2CH2 OH Pentan-1-ol (Position isomer)
CH3 CH2 CH CH3
OH Pentan-2-ol (Position isomer)
CH3 CH2 CH CH2 CH3
OH Pentan-3-ol (Position isomer)
CH3
CH3 CH2 CH2 C CH3
OH 2-methylbutan-2-ol (Position /structural isomer)
CH3
CH3 CH2 CH2 C CHOH
CH3 2,2-dimethylbutan-1-ol (Position /structural isomer)
CH3
CH3 CH2 CH C CH3
CH3 OH 2,3-dimethylbutan-1-ol (Position /structural isomer)
(iv)1,2-dichloropropan-2-ol
CClH2 CCl CH3
OH
(v)1,2-dichloropropan-1-ol
CClH2 CHCl CH2
OH
(vi) Ethan1,2-diol
H H
HOCH2CH2OH H-O – C – C – O-H
H H
(vii) Propan1,2,3-triol H OH H
HOCH2CHOHCH2OH H-O – C- C – C – O-H
H H H
C. LABORATORY PREPARATION OF ALKANOLS.
For decades the world over, people have been fermenting grapes juice, sugar, carbohydrates and starch to produce ethanol as a social drug for relaxation.
In large amount, drinking of ethanol by mammals /human beings causes mental and physical lack of coordination.
Prolonged intake of ethanol causes permanent mental and physical lack of coordination because it damages vital organs like the liver.
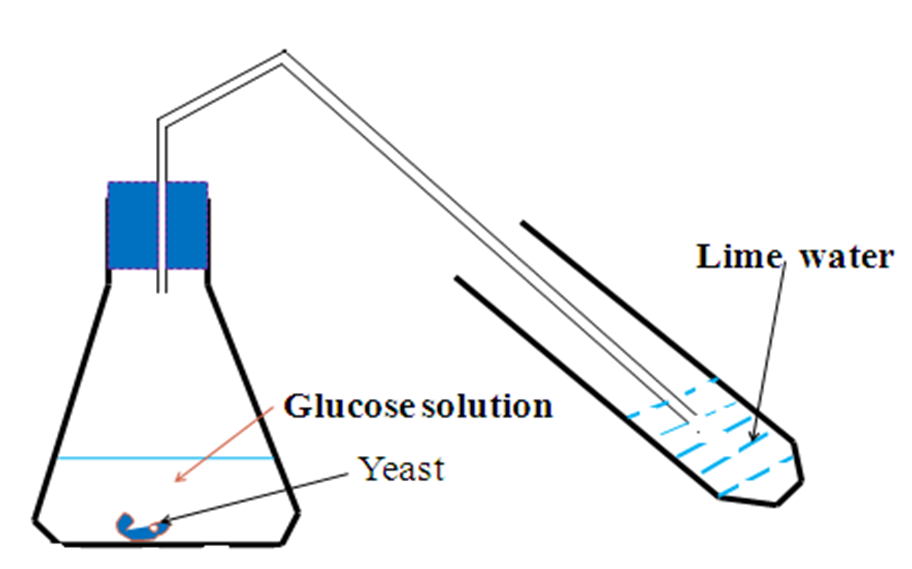
Fermentation is the reaction where sugar is converted to alcohol/alkanol using biological catalyst/enzymes in yeast.
It involves three processes:
(i)Conversion of starch to maltose using the enzyme diastase.
(C6H10O5)n (s) + H2O(l) –diastase enzyme –> C12H22O11(aq)
(Starch) (Maltose)
(ii)Hydrolysis of Maltose to glucose using the enzyme maltase.
C12H22O11(aq)+ H2O(l) — maltase enzyme –>2 C6H12O6(aq)
(Maltose) (glucose)
(iii)Conversion of glucose to ethanol and carbon(IV)oxide gas using the enzyme zymase.
C6H12O6(aq) — zymase enzyme –> 2 C2H5OH(aq) + 2CO2(g)
(glucose) (Ethanol)
At concentration greater than 15% by volume, the ethanol produced kills the yeast enzyme stopping the reaction.
To increases the concentration, fractional distillation is done to produce spirits (e.g. Brandy=40% ethanol).
Methanol is much more poisonous /toxic than ethanol.
Taken large quantity in small quantity it causes instant blindness and liver, killing the consumer victim within hours.
School laboratory preparation of ethanol from fermentation of glucose
Measure 100cm3 of pure water into a conical flask.
Add about five spatula end full of glucose.
Stir the mixture to dissolve.
Add about one spatula end full of yeast.
Set up the apparatus as below.