(a)Nomenclature/Naming of Alkynes
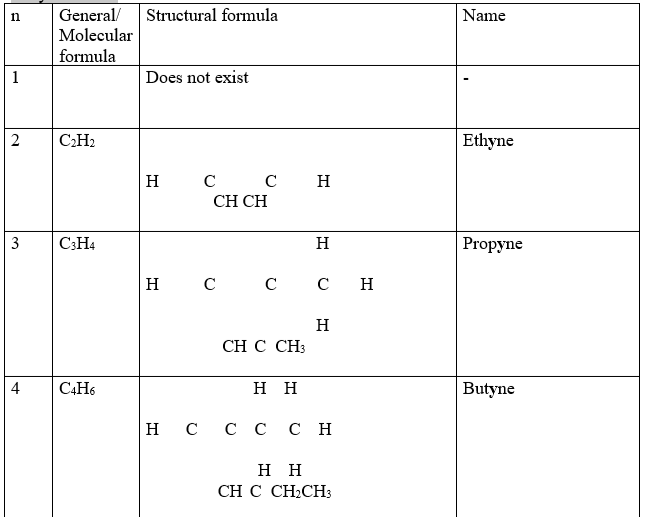
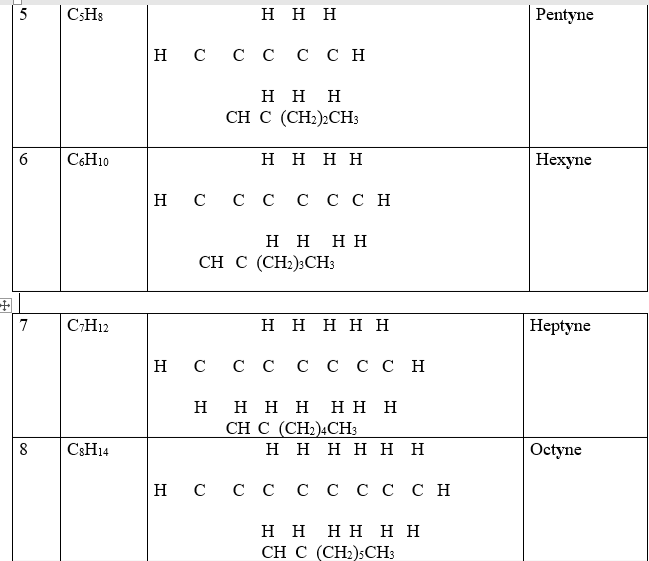
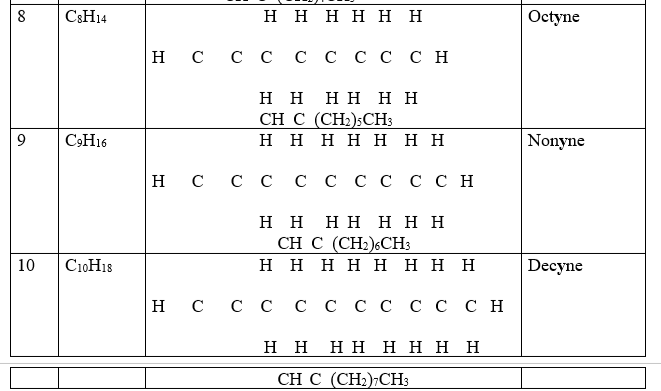
These are hydrocarbons with a general formula CnH2n–2 and C C double bond as the functional group . n is the number of Carbon atoms in the molecule.
The carbon atoms are linked by at least one triple bond to each other and single bonds to hydrogen atoms.
They include:
Note
1. Since carbon is tetravalent ,each atom of carbon in the alkyne MUST always be bonded using four covalent bond /four shared pairs of electrons including at the triple bond.
2. Since Hydrogen is monovalent ,each atom of hydrogen in the alkyne MUST always be bonded using one covalent bond/one shared pair of electrons.
3. One member of the alkyne ,like alkenes and alkanes, differ from the next/previous by a CH2 group(molar mass of 14 atomic mass units).They thus form a homologous series.
e.g
Propyne differ from ethyne by (14 a.m.u) one carbon and two Hydrogen atoms from ethyne.
4.A homologous series of alkenes like that of alkanes:
(i) differ by a CH2 group from the next /previous consecutively
(ii) have similar chemical properties
(iii)have similar chemical formula with general formula CnH2n-2
(iv)the physical properties also show steady gradual change
5.The – C = C – triple bond in alkyne is the functional group. The functional group is the reacting site of the alkynes.
6. The – C = C – triple bond in alkyne can easily be broken to accommodate more /four more monovalent atoms. The – C = C – triple bond in alkynes make it thus unsaturated like alkenes.
7. Most of the reactions of alkynes like alkenes take place at the – C = C- triple bond.
(b)Isomers of alkynes
Isomers of alkynes have the same molecular general formula but different molecular structural formula.
Isomers of alkynes are also named by using the IUPAC(International Union of Pure and Applied Chemistry) system of nomenclature/naming.
The IUPAC system of nomenclatureof naming alkynesuses the following basic rules/guidelines:
1.Identify the longest continuous/straight carbon chain which contains the – C = C- triple bond to get/determine the parent alkene.
2. Number the longest chain form the end of the chain which contains the -C = C- triple bond so as – C = C- triple bond get lowest number possible.
3 Indicate the positions by splitting “alk-positions-yne” e.g. but-2-yne, pent-1,3-diyne.
4.The position indicated must be for the carbon atom at the lower position in the
-C = C- triple bond. i.e
But-2-yne means the triple -C = C- is between Carbon “2”and “3”
Pent-1,3-diyne means there are two triple bonds; one between carbon “1” and “2”and another between carbon “3” and “4”
5. Determine the position, number and type of branches. Name them as methyl, ethyl, propyl e.tc. according to the number of alkyl carbon chains attached to the alkyne. Name them fluoro-,chloro-,bromo-,iodo- if they are halogens
6.Use prefix di-,tri-,tetra-,penta-,hexa- to show the number of triple – C = C- bonds and branches attached to the alkyne.
7.Position isomers can be formed when the – C = C- triple bond is shifted between carbon atoms e.g.
But-2-yne means the double – C = C- is between Carbon “2”and “3”
But-1-yne means the double – C = C- is between Carbon “1”and “2”
Both But-1-yne and But-2-yne are position isomers of Butyne.
9. Like alkanes and alkynes , an alkyl group can be attached to the alkyne. Chain/branch isomers are thus formed.
Butyne and 2-methyl propyne both have the same general formular but different branching chain.
(c)Preparation of Alkynes.
Ethyne is prepared from the reaction of water on calcium carbide. The reaction is highly exothermic and thus a layer of sand should be put above the calcium carbide to absorb excess heat to prevent the reaction flask from breaking. Copper(II)sulphate(VI) is used to catalyze the reaction
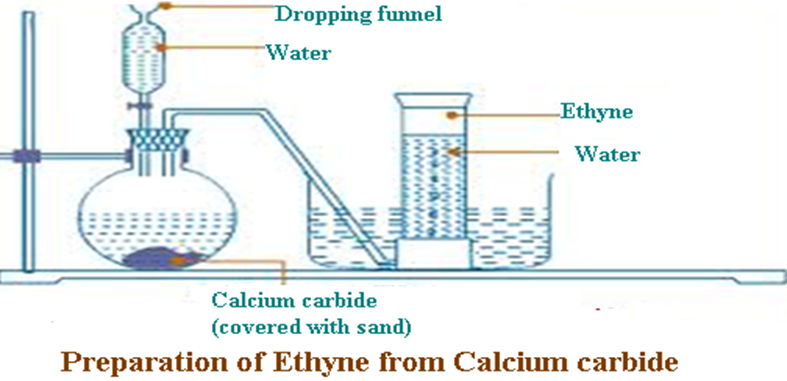
Chemical equation
CaC2(s) + 2 H2O(l) -> Ca(OH) 2 (aq) + C2H2 (g)
(d)Properties of alkynes
I. Physical properties
Like alkanes and alkenes, alkynes are colourles gases, solids and liquids that are not poisonous.
They are slightly soluble in water. The solubility in water decrease as the carbon chain and as the molar mass increase but very soluble in organic solvents like tetrachloromethane and methylbenzene. Ethyne has a pleasant taste when pure.
The melting and boiling point increase as the carbon chain increase.
This is because of the increase in van-der-waals /intermolecular forces as the carbon chain increase. The 1st three straight chain alkynes (ethyne,propyne and but-1-yne)are gases at room temperature and pressure.
The density of straight chain alkynes increase with increasing carbon chain as the intermolecular forces increases reducing the volume occupied by a given mass of the alkyne.
Summary of physical properties of the 1st five alkenes
| Alkyne | General formula | Melting point(oC) | Boiling point(oC) | State at room(298K) temperature and pressure atmosphere (101300Pa) |
| Ethyne | CH CH | -82 | -84 | gas |
| Propyne | CH3 C CH | -103 | -23 | gas |
| Butyne | CH3CH2 CCH | -122 | 8 | gas |
| Pent-1-yne | CH3(CH2) 2 CCH | -119 | 39 | liquid |
| Hex-1-yne | CH3(CH2) 3C CH | -132 | 71 | liquid |
II. Chemical properties
(a)Burning/combustion
Alkynes burn with a yellow/ luminous very sooty/ smoky flame in excess air to form carbon(IV) oxide and water.
Alkyne + Air -> carbon(IV) oxide + water (excess air/oxygen)
Alkenes burn with a yellow/ luminous verysooty/ smoky flame in limited air to form carbon(II) oxide/carbon and water.
Alkyne + Air -> carbon(II) oxide /carbon + water (limited air)
Burning of alkynes with a yellow/ luminous sooty/ smoky flame is a confirmatory test for the presence of the – C = C – triple bond because they have very high C:H ratio.
Examples of burning alkynes
1.(a) Ethyne when ignited burns with a yellow very sooty flame in excess air to form carbon(IV) oxide and water.
Ethyne + Air -> carbon(IV) oxide + water (excess air/oxygen)
2C2H2(g) + 5O2(g) -> 4CO2(g) + 2H2O(l/g)
(b) Ethyne when ignited burns with a yellow sooty flame in limited air to form a mixture of unburnt carbon and carbon(II) oxide and water.
Ethyne + Air -> carbon(II) oxide + water (limited air )
C2H2(g) + O2(g) -> 2CO2(g) + C + 2H2O(l/g)
2.(a) Propyne when ignited burns with a yellow sooty flame in excess air to form carbon(IV) oxide and water.
Propyne + Air -> carbon(IV) oxide + water (excess air/oxygen)
C3H4(g) + 4O2(g) -> 3CO2(g) + 2H2O(l/g)
(a) Propyne when ignited burns with a yellow sooty flame in limited air to form carbon(II) oxide and water.
Propene + Air -> carbon(IV) oxide + water (excess air/oxygen)
2C3H4(g) + 5O2(g) -> 6CO(g) + 4H2O(l/g)
(b)Addition reactions
An addition reaction is one which an unsaturated compound reacts to form a saturated compound. Addition reactions of alkynes are also named from the reagent used to cause the addition/convert the triple – C = C- to single C- C bond.
(i)Hydrogenation
Hydrogenation is an addition reaction in which hydrogen in presence of Palladium/Nickel catalyst at 150oC temperatures react with alkynes to form alkenes then alkanes.
Examples
1.During hydrogenation, two hydrogen atom in the hydrogen molecule attach itself to one carbon and the other two hydrogen to the second carbon breaking the triple bond to double the single.
Chemical equation
HC = CH + H2 -Ni/Pa -> H2C = CH2 + H2 -Ni/Pa -> H2C – CH2
H H H H H H
C = C + H – H – Ni/Pa -> H – C = C – H + H – H – Ni/Pa -> H – C – C – H
H H H H H H
2.Propyne undergo hydrogenation to form Propane
Chemical equation
H3C CH = CH2 + 2H2 -Ni/Pa-> H3C CH – CH3
H H H H H H
H C C = C + 2H – H – Ni/Pa-> H – C – C – C- H
H H H H H
3(a) But-1-yne undergo hydrogenation to form Butane
Chemical equation
But-1-yne + Hydrogen –Ni/Pa-> Butane
H3C CH2 C = CH + 2H2 -Ni/Pa-> H3C CH2CH – CH3
H H H H H H H
H C C – C = C + 2H – H – Ni/Pa-> H – C- C – C – C- H
H H H H H H
(b) But-2-yne undergo hydrogenation to form Butane
Chemical equation
But-2-yne + Hydrogen –Ni/Pa-> Butane
H3C C = C CH2 + 2H2 -Ni/Pa-> H3C CH2CH – CH3
H H H H H H
H C C = C – C H + 2H – H- Ni/Pa-> H – C- C – C – C- H
H H H H H H
(ii) Halogenation.
Halogenation is an addition reaction in which a halogen (Fluorine, chlorine, bromine, iodine) reacts with an alkyne to form an alkene then alkane.
The reaction of alkynes with halogens with alkynes is faster than with alkenes. The triple bond in the alkyne break and form a double then single bond.
The colour of the halogen fades as the number of moles of the halogens remaining unreacted decreases.
Two bromine atoms bond at the 1st carbon in the triple bond while the other two goes to the 2nd carbon.
Examples
1Ethyne reacts with brown bromine vapour to form 1,1,2,2-tetrabromoethane.
Chemical equation
HC = CH + 2Br2 H Br2 C – CH Br2
H H H H
C = C + 2Br – Br Br – C – C – Br
Br Br
Ethyne + Bromine 1,1,2,1-tetrabromoethane
2.Propyne reacts with chlorine to form 1,1,2,2-tetrachloropropane.
Chemical equation
H3C C = CH + 2Cl2 H3C CHCl2 – CHCl2
Propyne + Chlorine 1,1,2,2-tetrachloropropane
H H Cl H
H C C = C + 2Cl – Cl H – C – C – C- Cl
H H H Cl Cl
Propyne + Iodine 1,1,2,2-tetraiodopropane
H3C C = CH + 2I2 H3C CHI2 – CHI2
H H H H H I H
H C C – C = C + 2I – I H – C- C – C – C- I
H H H H I I
3(a)But-1-yne undergo halogenation to form 1,1,2,2-tetraiodobutane with iodine
Chemical equation
But-1-yne + iodine 1,1,2,2-tetrabromobutane
H3C CH2 C = CH + 2I2 H3C CH2C I2 – CHI2
H H H H I I
H C C – C = C -H + 2I – I H – C- C – C – C- H
H H H H H I I
(b) But-2-yne undergo halogenation to form 2,2,3,3-tetrafluorobutane with fluorine But-2-yne + Fluorine 2,2,3,3-tetrafluorobutane
H3C C = C -CH2 + 2F2 H3C CF2CF2 – CH3
H H H H H H H H
H C C = C – C -H + F – F H – C- C – C – C- H
H H H H H H
4. But-1,3-diyne should undergo halogenation to form 1,1,2,3,3,4,4 octaiodobutane. The reaction uses four moles of iodine molecules/eight iodine atoms to break the two(2) triple double bonds at carbon “1” and “2”.
But-1,3-diene + iodine 1,2,3,4-tetraiodobutane
H C = C C = C H + 4I2 H C I2 C I2 C I2 C H I2
I I I I
H C C – C = C -H + 4(I – I) H – C- C – C – C- H
I I I I
(iii) Reaction with hydrogen halides.
Hydrogen halides reacts with alkyne to form a halogenoalkene then halogenoalkane. The triple bond in the alkyne break and form a double then single bond.
The main compound is one which the hydrogen atom bond at the carbon with more hydrogen .
Examples
1. Ethyne reacts with hydrogen bromide to form bromoethane.
Chemical equation
H C = C H + 2HBr H3 C – CH Br2
H H H H
C = C + 2H – Br H – C – C – Br
H Br
Ethyne + Bromine 1,1-dibromoethane
2. Propyne reacts with hydrogen iodide to form 2,2-diiodopropane (as the main product )
Chemical equation
H3C C = CH + 2HI H3C CHI2 – CH3
| Carbon atom with more Hydrogen atoms gets extra hydrogen |
Propene + Chlorine 2,2-dichloropropane
H H I H
H C C = C + 2H – I H – C – C – C– H
H H H I H
3. Both But-1-yne and But-2-yne reacts with hydrogen bromide to form 2,2- dibromobutane
Chemical equation
But-1-ene + hydrogen bromide 2,2-dibromobutane
H3C CH2 C = CH + 2HBr H3C CH2CHBr -CH3
H H H H Br H
H C C – C = C + 2H – Br H – C- C – C – C- H
H H H H H Br H
But-2-yne + Hydrogen bromide 2,2-dibromobutane
H3C C = C -CH3 + 2HBr H3C CBr2CH2 – CH3
H H H Br H H
H C C = C – C -H + 2Br – H H – C- C – C – C- H
H H H Br H H
4. But-1,3-diene react with hydrogen iodide to form 2,3- diiodobutane. The reaction uses four moles of hydrogen iodide molecules/four iodine atoms and two hydrogen atoms to break the two double bonds.
But-1,3-diyne + iodine 2,2,3,3-tetraiodobutane
H C = C C = C H + 4HI H3C C I2 C I2 CH3
H H H I I H
H C C – C = C -H + 4(H – I) H – C- C – C – C- H
H I I H
