The periodic table does not classify elements as metals and non-metals. The table arranges them in terms of atomic numbers.
However, based on structure and bonding of the elements in the periodic table;
(i)-the top right hand corner of about twenty elements is non-metals
(ii)-left of each non-metal is an element which shows characteristics of both metal and non-metal.
These elements are called semi-metals/metalloids. They include Boron, silicon, Germanium, Arsenic, and Terullium
(iii)-all other elements in the periodic table are metal.
(iv)-Hydrogen is a non-metal with metallic characteristic/property of donating/losing outer electron to form cation/H+ ion.
(v) –bromine is the only known natural liquid non-metal element at room temperature and pressure.
(vi) –mercury is only known natural liquid metal element at room temperature and pressure.
(vii) Carbon-graphite is a semi metals/metalloids. Carbon-diamond is a pure non-metal yet both are allotropes of carbon (same element)
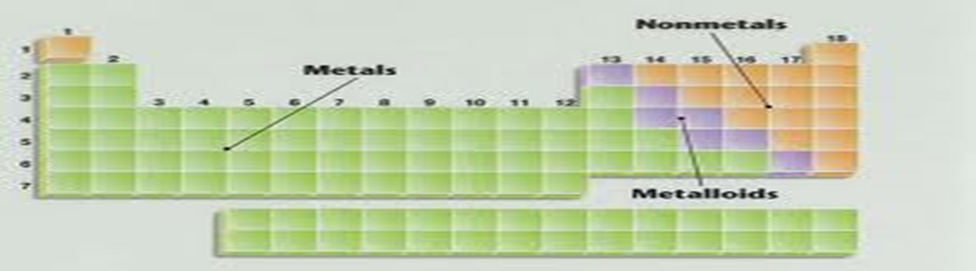
a) Sketch of the periodic table showing metals ,metalloid and non-metals
Metals Metalloids Non-metals
| H | He | |||||||
| Li | Be | B | C | N | O | F | Ne | |
| Na | Mg | Al | Si | P | S | Cl | Ar | |
| K | Ca | Transition metals | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | In | Sn | Sb | Te | I | Xe | |
| Cs | Ba | Tl | Pb | Bi | Po | At | Rn | |
| Fr | Ra |
b)Periodicity in the physical properties of elements across period 2 and 3
Study table I and II below:
Table I(period 2)
| Property | Li | Be | B | C | N | O | F | Ne |
| Melting point(oC) | 180 | 1280 | 2030 | 3700 (graphite) 3550 (diamond) | -210 | -219 | -220 | -250 |
| Boiling point(oC) | 1330 | 2480 | 3930 | Graphite sublimes 4830 (diamond) | -200 | -180 | -190 | -245 |
| Density at room temperature (gcm-3) | 0.50 | 1.85 | 2.55 | 2.25 (graphite) 3.53 (diamond) | 0.81 | 0.14 | 0.11 | 0.021 |
| Type of element | Metal | Metal | Metal | Metalloid | Non-metal | Non-metal | Non-metal | Non-metal |
| Chemical structure | Giant metallic | Giant metallic | Giant atomic/ covalent | Giant atomic/ covalent | Simple molecula or molecule/ N2 | Simple molecula or molecules /O2 | Simple molecula or molecule/F2 | Simple molecula or molecule/Ne |
| State at room temperature | Solid | Solid | Solid | Solid | gas | gas | gas | gas |
| Electron structure | 2:1 | 2:2 | 2:3 | 2:4 | 2:5 | 2:6 | 2:7 | 2:8 |
| Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | – |
| Formular of ion | Li+ | Be2+ | B3+ | – | N3- | O2- | F– | – |
Table II (period 3)
| Property | Na | Mg | Al | Si | P(white) | S(Rhombic) | Cl | Ar |
| Melting point(oC) | 98 | 650 | 660 | 1410 | 44 | 114 | -101 | -189 |
| Boiling point(oC) | 890 | 1120 | 2450 | 2680 | 280 | 445 | -34 | -186 |
| Density at room temperature (gcm-3) | 0.97 | 1.74 | 2.70 | 2.33 (graphite) 3.53 (diamond) | 1.82 | 2.07 | 0.157 | 0.011 |
| Type of element | Metal | Metal | Metal | Metalloid | Non-metal | Non-metal | Non-metal | Non-metal |
| Chemical structure | Giant metallic | Giant metallic | Giant metallic | Giant atomic/ covalent | Simple molecula or molecule/ P4 | Simple molecula or molecules /S8 | Simple molecula or molecule/Cl2 | Simple molecula or molecule/Ar |
| State at room temperature | Solid | Solid | Solid | Solid | Solid | Solid | gas | gas |
| Electron structure | 2:8:1 | 2:8:2 | 2:8:3 | 2:8:4 | 2:8:5 | 2:8:6 | 2:8:7 | 2:8:8 |
| Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | – |
| Formular of ion | Na+ | Mg2+ | Al3+ | – | P3- | S2- | Cl– | – |
From table I and II above:
1. Explain the trend in atomic radius along /across a period in the periodic table
Observation
Atomic radius of elements in the same period decrease successively across/along a period from left to right.
Explanation
Across/along the period from left to right there is an increase in nuclear charge from additional number of protons and still additional number of electrons entering the same energy level.
Increase in nuclear charge increases the effective nuclear attraction on the outer energy level pulling it closer to the nucleus successively across the period .e.g.
(i)From the table 1and 2 above, atomic radius of Sodium (0.157nM) is higher than that of Magnesium(0.137nM). This is because Magnesium has more effective nuclear attraction on the outer energy level than Sodium hence pulls outer energy level more nearer to its nucleus than sodium.
(ii)The rate of decrease in the atomic radius become smaller as the atom become heavier e.g. Atomic radius of Magnesium from sodium falls by(0.157nM- 0.137nM) =0.02
Atomic radius of Chlorine from sulphur falls by(0.104nM- 0.099nM) =0.005
This is because gaining/adding one more proton to 11 already present cause greater proportional change in nuclear attraction power to magnesium than gaining/adding one more proton to 16 already present in sulphur to chlorine.
(iii)Period 3 elements have more energy levels than Period 2 elements. They have therefore bigger/larger atomic radius/size than corresponding period 2 elements in the same group.
2. Explain the trend in ionic radius along/across a period in the periodic table
Observation
Ionic radius of elements in the same period decrease successively across/along a period from left to right for the first three elements then increase drastically then slowly successively decrease.
Explanation
Across/along the period from left to right elements change form electron donors/losers (reducing agents) to electron acceptors (oxidizing agents).
(i)An atom form stable ion by either gaining/acquiring/ accepting extra electron or donating/losing outer electrons.
(ii)Metals form stable ions by donating/losing all the outer energy level electrons and thus also the outer energy level .i.e.
-Sodium ion has one less energy level than sodium atom. The ion is formed by sodium atom donating/losing (all) the outer energy level electron and thus also the outer energy level making the ion to have smaller ionic radius than atom.
(iii)Ionic radius therefore decrease across/along the period from Lithium to Boron in period 2 and from Sodium to Aluminium in period 3.This is because the number of electrons donated/lost causes increased effective nuclear attraction on remaining electrons /energy levels.
(iv) Non-metals form stable ion by gaining/acquiring/accepting extra electron in the outer energy level. The extra electron/s increases the repulsion among electrons and reduces the effective nuclear attraction on outer energy level. The outer energy level therefore expand/enlarge/increase in order to accommodate the extra repelled electrons .The more electrons gained/accepted/acquired the more repulsion and the more expansion to accommodate them and hence bigger/larger atomic radius. e.g.
–Nitrogen ion has three electrons more than Nitrogen atom. The outer energy level expand/enlarge/increase to accommodate the extra repelled electrons. Nitrogen atom thus has smaller atomic radius than the ionic radius of nitrogen ion.
(v) Ionic radius decrease from group IV onwards from left to right. This because the number of electrons gained to form ion decrease across/along the period from left to right. e.g. Nitrogen ion has bigger/larger ionic radius than Oxygen.
3. Explain the trend in melting and boiling point of elements in a period in the periodic table.
Observation
The melting and boiling point of elements rise up to the elements in Group IV(Carbon/Silicon) along/across the period then continuously falls.
Explanation
Melting/boiling points depend on the packing of the structure making the element and the strength of the bond holding the atoms/molecules together.
Across/along the period (2 and 3) the structure changes from giant metallic, giant atomic/covalent to simple molecular.
(i)For metals, the number of delocalized electrons increases across/along the period and hence stronger metallic bond/structure thus requiring a lot of heat/energy to weaken.
The strength of a metallic bond also depends on the atomic radius/size. The melting /boiling point decrease as the atomic radius/size of metals increase due to decreased packing of larger atoms. e.g.
-The melting /boiling point of Lithium is lower than that of Beryllium because Beryllium has two/more delocalized electrons and hence stronger metallic structure/bond.
– The melting /boiling point of Lithium is higher than that of Sodium because the atomic radius/size Lithium is smaller and hence better packed and hence forms stronger metallic structure/bond.
(ii)Carbon-graphite/carbon-diamond in period 2 and Silicon in period 3 form very well packed giant atomic/covalent structures held together by strong covalent bonds. These elements have therefore very high melting/boiling points.
Both Carbon-graphite/ carbon-diamond have smaller atomic radius/size than Silicon in period 3 and thus higher melting/boiling points due to better/closer packing of smaller atoms in their well packed giant atomic/covalent structures.
(ii) Non-metals from group V along/across the period form simple molecules joined by weak intermolecular /van-der-waals force. The weak intermolecular /van-der-waals force require little energy/heat to weaken leading to low melting/boiling points. The strength of the intermolecular /van-der-waals forces decrease with decrease in atomic radius/ size lowering the melting/boiling points along/across the period (and raising the melting/boiling points down the group).e.g.
-The melting /boiling point of Nitrogen is higher than that of Oxygen. This is because the atomic radius/ size of Nitrogen is higher than that of Oxygen and hence stronger intermolecular /van-der-waals forces between Nitrogen molecules.
-The melting /boiling point of Chlorine is higher than that of Fluorine. This is because the atomic radius/ size of Chlorine is higher than that of Fluorine and hence stronger intermolecular /van-der-waals forces between Chlorine molecules.
(iii) Rhombic sulphur exists as a puckered ring of S8atoms which are well packed. Before melting the ring break and join to very long chains that entangle each other causing the unusually high melting/boiling point of Rhombic sulphur.
(iv) Both sulphur and phosphorus exists as allotropes.
Sulphur exists as Rhombic-sulphur and monoclinic-sulphur. Rhombic-sulphur is the stable form of sulphur at room temperature and pressure.
Phosphorus exists as white-phosphorus and red-phosphorus.
White-phosphorus is the stable form of Phosphorus at room temperature and pressure.
4. State and explain the trend in density of elements in a period in the periodic table.
Observation: Density increase upto the elements in group IV then falls across/along the period successively
Explanation:
Density is the mass per unit volume occupied by matter/particles/atoms/molecules of element.
(i)For metals ,the stronger metallic bond and the more delocalized electrons ensure a very well packed giant metallic structure that occupy less volume and thus higher density.The more the number of delocalized electrons along/across the period, the higher the density. e.g.
(i)Aluminium has a higher density than sodium. This is because aluminium has more /three delocalized electrons than /one sodium thus forms a very well packed giant metallic structure that occupy less volume per given mass/density.
(ii)Carbon-graphite ,carbon-diamond and silicon in group IV form a well packed giant atomic/covalent structure that is continuously joined by strong covalent bonds hence occupy less volume per given mass/density.
Carbon-graphite form a less well packed giant hexagonal planar structure joined by Van-der-waals forces. Its density (2.25gcm-3) is therefore less than that of Carbon-diamond(3.53gcm-3) and silicon(2.33gcm-3).Both diamond and silicon have giant tetrahedral structure that is better packed. Carbon-diamond has smaller atomic radius/size than silicon. Its density is thus higher because of better packing and subsequently higher density. Carbon-diamond is the hardest known natural substance by having the highest density.
(iii) For non-metals, the strength of the intermolecular /van-der-waals forces decreases with decrease in atomic radius/size along/across the period. This decreases the mass occupied by given volume of atoms in a molecule from group VI onwards. e.g.
Phosphorus has a higher atomic radius/size than chlorine and Argon and thus stronger intermolecular/van-der-waals forces that ensure a given mass of phosphorus occupy less volume than chlorine and neon.
5. State and explain the trend in thermal/electrical conductivity of elements in a period in the periodic table.
Observation:
Increase along/across the period from group I, II, and III then decrease in Group IV to drastically decrease in group V to VIII (O).
Explanation
(i)Metals have free delocalized electrons that are responsible for thermal/electrical conductivity.Thermal/electrical conductivity increase with increase in number of delocalized electrons. The thermal conductivity decrease with increase in temperature/heating.e.g.Aluminium with three delocalized electrons from each atom in its metallic structure has the highest electrical /thermal conductivity in period 3.
(ii)Carbon-graphite has also free 4th valency electrons that are delocalized within its layers of giant hexagonal planar structure. They are responsible for the electrical conductivity of graphite.
(iii)Silicon and carbon diamond do not conduct electricity but conducts heat. With each atom too close to each other in their very well packed giant tetrahedral structure, heat transfer /radiate between the atoms. The thermal conductivity increase with increase in temperature/heating.
(iv) All other non-metals are poor /non-conductor of heat and electricity. They are made of molecules with no free /mobile delocalized electrons in their structure.
Periodicity of the oxides of elements along/across period 3
The table below summarizes some properties of the oxides of elements in period 3 of the periodic table.
| Formular of oxide/ Property | Na2O | MgO | Al2O3 | SiO2 | P2O5 P4O6 | SO2 SO3 | Cl2O7 Cl2O |
| Melting point(oC) | 1193 | 3075 | 2045 | 1728 | 563 | -76 | -60 |
| Boiling point(oC) | 1278 | 3601 | 2980 | 2231 | 301 | -10 | -9 |
| Bond type | Ionic | Ionic | Ionic | Covalent | Covalent | Covalent | Covalent |
| Chemical structure | Giant ionic structure | Giant ionic structure | Giant ionic structure | Giant atomic/ covalent | Simple molecula or molecule | Simple molecula or molecules | Simple molecula or molecule |
| State at room Temperature | Solid | Solid | Solid | Solid | Solid | gas | Gas (Cl2O7 is a liquid) |
| Nature of Oxide | Basic/ alkaline | Basic/ alkaline | Amphotellic oxide | 2:8:4 | 2:8:5 | 2:8:6 | 2:8:7 |
| Reaction with water | React to form NaOH /alkaline solution | React to form MgOH)2 /weakly alkaline solution | Don’t react with water. | Don’t react with water. | React to form H2PO4 /weakly acidic solution | -SO2 react to form H2SO3 . H2SO3 is quickly oxidized to H2SO4 -SO2 react to form H2SO4/ strongly acidic | -Cl2O7 reacts to form HClO4 /weakly acidic solution |
| Reaction with dilute acids | Reacts to form salt and water | Reacts to form salt and water | Reacts to form salt and water | No reaction | No reaction | No reaction | No reaction |
1. All the oxides of elements in period 3 except those of sulphur and chlorine are solids at room temperature and pressure.
2. Across/along the period, bonding of the oxides changes from ionic in sodium oxide magnesium oxide and aluminium oxide (show both ionic and covalent properties) to covalent in the rest of the oxides.
3. Across/along the period, the structure of the oxides changes from giant ionic structure in sodium oxide, magnesium oxide and aluminium oxide to giant atomic/covalent structure in silicon (IV) oxide. The rest of the oxides form simple molecules/molecular structure.
4. Sodium oxide and magnesium oxide are basic /alkaline in nature. Aluminium oxide is amphotellic in nature (shows both acidic and basic characteristics). The rest of the oxides are acidic in nature.
5. Ionic compounds/oxides have very high melting/boiling points because of the strong electrostatic attraction joining the giant ionic crystal lattice.
The melting/boiling points increase from sodium oxide to aluminium oxide as the number of electrons involved in bonding increase, increasing the strength of the ionic bond/structure.
6. Silicon (IV) oxide is made of a well packed giant atomic/covalent structure joined by strong covalent bonds.
This results in a solid with very high melting/boiling point.
7.Phosphorus (V) oxide, sulphur(IV) oxide/ sulphur (VI) oxide and dichloride heptoxide exist as simple molecules/molecular structure joined by weak van-der-waals/intermolecular forces.
This results in them existing as low melting /boiling point solids/gases.
8. Ionic oxide conducts electricity in molten and aqueous states but not in solid.
In solid state the ions are fused/fixed but on heating to molten state and when dissolved in water, the ions are free / mobile.
Sodium oxide, magnesium oxide and aluminium oxide are therefore good conductors in molten and aqueous states.
9. Covalent bonded oxides do not conduct electricity in solid, molten or in aqueous states.
This is because they do not have free / mobile ion. Phosphorus (V) oxide, sulphur (IV) oxide/ sulphur (VI) oxide and dichloride heptoxide are thus non-conductors/insulators.
10. Silicon (IV) oxide is a poor/weak conductor of heat in solid state. This is because it has very closely packed structure for heat to radiate conduct along its structure.
11. Electopositivity decrease across the period while electronegativity increase across the period. The oxides thus become less ionic and more covalent along/across the period.
12.The steady change from giant ionic structure to giant atomic/ covalent structure then simple molecular structure lead to profound differences in the reaction of the oxides with water,acids and alkalis/bases:
(i) Reaction with water
a) Ionic oxides react with water to form alkaline solutions e.g.;
I.Sodium oxide reacts/dissolves in water forming an alkaline solution of sodium hydroxide.
Chemical equation: Na2O(s) + H2O (l) -> 2NaOH(aq)
II. Magnesium oxide slightly/ slowly reacts/dissolves in water forming an alkaline solution of magnesium hydroxide
Chemical equation: MgO(s) + 2H2O (l) -> Mg(OH) 2 (aq)
III. Aluminium oxide does reacts/dissolves in water.
b) Non-metallic oxides are acidic. They react with water to form weakly acidic solutions:
I. Phosphorus (V) oxide readily reacts/dissolves in water forming a weak acidic solution of phosphoric (V) acid.
Chemical equation: P4O10 (s) + 6H2O (l) -> 4H3PO4 (aq)
Chemical equation: P2O5 (s) + 3H2O (l) -> 2H3PO4 (aq)
II. Sulphur (IV) oxide readily reacts/dissolves in water forming a weak acidic solution of sulphuric (IV) acid.
Chemical equation: SO2 (g) + H2O (l) -> H2SO3 (aq)
Sulphur (VI) oxide quickly fumes in water to form concentrated sulphuric (VI) acid which is a strong acid.
Chemical equation: SO3 (g) + H2O (l) -> H2SO4 (aq)
III. Dichlorine oxide reacts with water to form weak acidic solution of chloric(I) acid/hypochlorous acid.
Chemical equation: Cl2O (g) + H2O (l) -> 2HClO (aq)
IV. Dichlorine heptoxide reacts with water to form weak acidic solution of chloric(VII) acid.
Chemical equation: Cl2O7 (l) + H2O (l) -> 2HClO4 (aq)
c) Silicon (IV) oxide does not react with water.
It reacts with hot concentrated alkalis forming silicate (IV) salts. e.g.
Silicon (IV) oxide react with hot concentrated sodium hydroxide to form sodium silicate (IV) salt.
Chemical equation: SiO2 (s) + 2NaOH (aq) -> Na2SiO3 (aq) + H2O (l)
(ii) Reaction with dilute acids
a) Ionic oxides react with dilute acids to form salt and water only. This is a neutralization reaction. e.g.
Chemical equation: Na2O(s) + H2SO4 (aq) -> Na2SO4 (aq) + H2O(l)
Chemical equation: MgO(s) + 2HNO3(aq) -> Mg (NO3) 2 (aq) + H2O(l)
Chemical equation: Al2O3 (s) + 6HCl(aq) -> 2AlCl3 (aq) + 3H2O(l)
Aluminium oxide is amphotellic and reacts with hot concentrated strong alkalis sodium/potassium hydroxides to form complex sodium aluminate(III) and potassium aluminate(III) salt.
Chemical equation: Al2O3 (s) + 2NaOH (aq) + 3H2O(l) -> 2 NaAl(OH)4 (aq)
Chemical equation: Al2O3 (s) + 2KOH(aq) + 3H2O(l) -> 2 KAl(OH)4 (aq)
b) Acidic oxides do not react with dilute acids.
c) Periodicity of the Chlorides of elements along/across period 3
The table below summarizes some properties of the chlorides of elements in period 3 of the periodic table.
| Formular of chloride/ Property | NaCl | MgCl2 | AlCl3 | SiCl4 | PCl5 PCl3 | SCl2 S2Cl2 | Cl2 |
| Melting point(oC) | 801 | 714 | Sublimes at 180 oC | -70 | PCl5 Sublimes at -94 oC | -78 | -101 |
| Boiling point(oC) | 1465 | 1418 | 423(as Al2Cl6 vapour | 57 | 74(as P2Cl6 Vapour 164 (as PCl5) | decomposes at 59 oC | -34 |
| Bond type | Ionic | Ionic | Ionic/ Covalent/ dative | Covalent | Covalent | Covalent | Covalent |
| Chemical structure | Giant ionic structure | Giant ionic structure | Molecular/ dimerizes | Simple molecula or molecule | Simple molecula or molecule | Simple molecula or molecules | Simple molecula or molecule |
| State at room Temperature | Solid | Solid | Solid | liquid | Liquid PCl5 is solid | liquid | Gas |
| Nature of Chloride | Neutral | Neutral | Strongly acidic | Strongly acidic | Strongly acidic | Strongly acidic | Strongly acidic |
| pH of solution | 7.0 | 7.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Reaction with water | Dissolve | Dissolve | -Hydrolysed by water -Acidic hydrogen chloride fumes produced | -Hydrolysed by water -Acidic hydrogen chloride fumes produced | Hydrolysed by water -Acidic hydrogen chloride fumes produced | Hydrolysed by water -Acidic hydrogen chloride fumes produced | Forms HCl and HClO |
| Electrical conductivity in molten/aqueous state | good | good | poor | nil | nil | nil | nil |
1. Sodium Chloride, Magnesium chloride and aluminium chloride are solids at room temperature and pressure.
Silicon(IV) chloride, phosphorus(III)chloride and disulphur dichloride are liquids. Phosphorus(V)chloride is a solid. Both chlorine and sulphur chloride are gases.
2. Across/along the period bonding changes from ionic in Sodium Chloride and Magnesium chloride to covalent in the rest of the chlorides.
3. Anhydrous aluminium chloride is also a molecular compound .Each aluminium atom is covalently bonded to three chlorine atoms.
In vapour/gaseous phase/state two molecules dimerizes to Al2O6 molecule through coordinate/dative bonding.
4. Across/along the period the structure changes from giant ionic in Sodium Chloride and Magnesium chloride to simple molecules/molecular structure in the rest of the chlorides.
5. Ionic chlorides have very high melting /boiling points because of the strong ionic bond/electrostatic attraction between the ions in their crystal lattice.The rest of the chlorides have low melting /boiling points because of the weak van-der-waal /intermolecular forces.
6. Sodium Chloride and Magnesium chloride in molten and aqueous state have free/mobile ions and thus good electrical conductors. Aluminium chloride is a poor conductor. The rest of the chlorides do not conduct because they have no free/mobile ions.
7. Ionic chloride form neutral solutions with pH =7. These chlorides ionize/dissociate completely into free cations and anions.i.e;
Sodium Chloride and Magnesium chloride have pH=7 because they are fully/completely ionized/dissociated into free ions.
Chemical equation NaCl (s) -> Na+(aq) + Cl–(aq) Chemical equation MgCl2 (s) -> Mg2+(aq) + 2Cl–(aq)
8 Across/along the period from aluminium chloride, hydrolysis of the chloride takes place when reacting/dissolved in water.
Hydrolysis is the reaction of a compound when dissolved in water.
a) Aluminium chloride is hydrolyzed by water to form aluminium hydroxide and fumes of hydrogen chloride gas. Hydrogen chloride gas dissolves in water to acidic hydrochloric acid. Hydrochloric acid is a strong acid with low pH and thus the mixture is strongly acidic.
Chemical equation AlCl3 (s) + 3H2O(l)-> Al(OH)3(s) + 3HCl(g)
b)Silicon(IV) chloride is hydrolyzed by water to form silicon(IV)oxide and fumes of hydrogen chloride gas. Hydrogen chloride gas dissolves in water to acidic hydrochloric acid. Hydrochloric acid is a strong acid with low pH and thus the mixture is strongly acidic.
Chemical equation SiCl4 (l) + 2H2O(l)-> SiO2(s) + 4HCl(g)
This reaction is highly exothermic producing /evolving a lot of heat that cause a rise in the temperature of the mixture.
c) Both phosphoric (V) chloride and phosphoric (III) chloride are hydrolyzed by water to form phosphoric (V) acid and phosphoric (III) acid respectively. Fumes of hydrogen chloride gas are produced. Hydrogen chloride gas dissolves in water to acidic hydrochloric acid. Hydrochloric acid is a strong acid with low pH and thus the mixture is strongly acidic.
Chemical equation PCl5 (s) + 4H2O(l)-> H3PO4(aq) + 5HCl(g)
Chemical equation PCl3 (s) + 3H2O(l)-> H3PO4(aq) + 3HCl(g)
This reaction is also highly exothermic producing /evolving a lot of heat that cause a rise in the temperature of the mixture.
d) Disulphur dichloride similarly hydrolyzes in water to form yellow deposits of sulphur and produce a mixture of sulphur (IV) oxide and hydrogen chloride gas. Hydrogen chloride gas dissolves in water to acidic hydrochloric acid. Hydrochloric acid is a strong acid with low pH and thus the mixture is strongly acidic.
Chemical equation 2S2Cl2 (l) + 2H2O(l)-> 3S(s) + SO2(g) + 4HCl(g)
