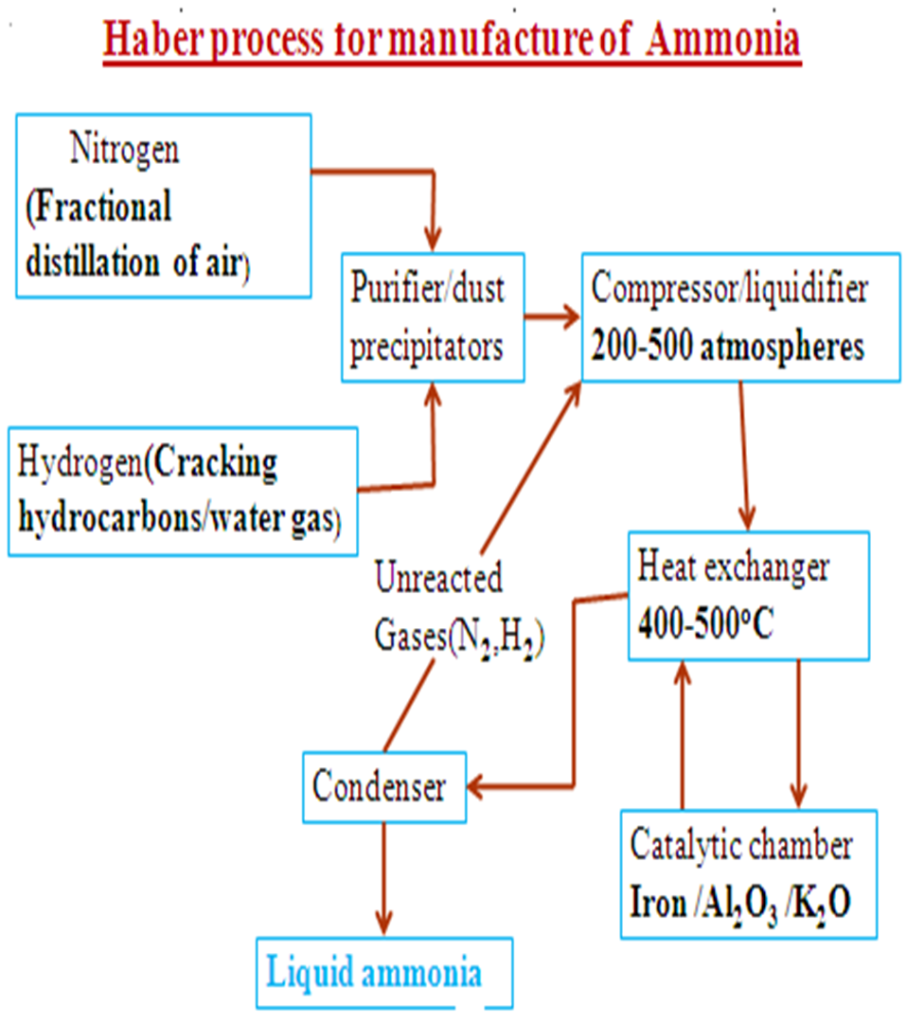
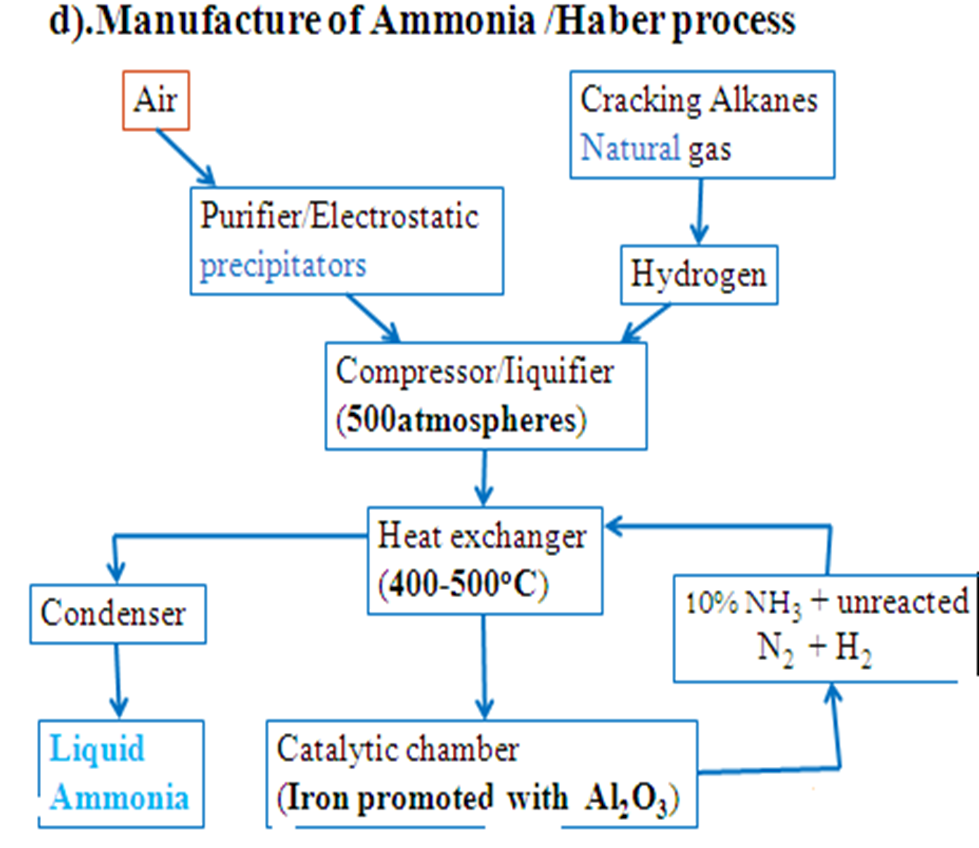
Optimum Conditions in Haber Processs
Chemical equation
N2 (g) + 3H2 (g) ===Fe/Pt=== 2NH3 (g) ΔH = -92kJ
Equilibrium/Reaction rate considerations
(i)Removing ammonia gas once formed shift the equilibrium forward to the right to replace the ammonia. More/higher yield of ammonia is attained.
(ii)Increase in pressure shift the equilibrium forward to the right where there is less volume/molecules. More/higher yield of ammonia is attained. Very high pressures raise the cost of production because they are expensive to produce and maintain. An optimum pressure of about 200atmospheres is normally used.
(iii)Increase in temperature shift the equilibrium backward to the left because the reaction is exothermic (ΔH = -92kJ) . Ammonia formed decomposes back to Nitrogen and Hydrogen to remove excess heat therefore a less yield of ammonia is attained. Very low temperature decreases the collision frequency of Nitrogen and Hydrogen and thus the rate of reaction too slow and uneconomical.
An optimum temperature of about 450oC is normally used.
(iv)Iron and platinum can be used as catalyst. Platinum is a better catalyst but more expensive and easily poisoned by impurities than Iron. Iron is promoted /impregnated with AluminiumOxide(Al2O3) to increase its surface area/area of contact with reactants and thus efficiency. The catalyst does not increase the yield of ammonia but it speed up its rate of formation.
