A.NITROGEN
a) Occurrence:
Nitrogen is found in the atmosphere occupying about 78% by volume of air.
Proteins, amino acids, polypeptides in living things contain nitrogen.
b) Isolation of nitrogen from the air.
Nitrogen can be isolated from other gases present in air like oxygen, water (vapour), carbon (IV) oxide and noble gases as in the school laboratory as in the flow chart below:
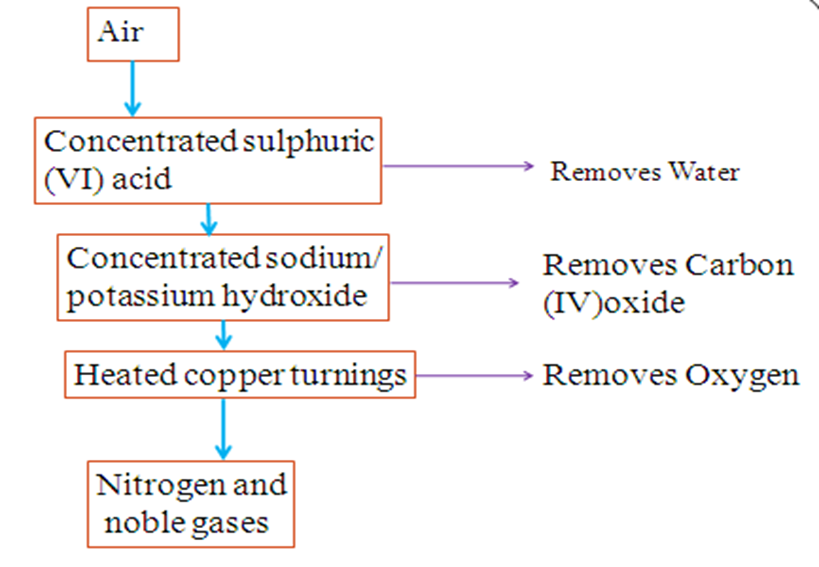
Water is added slowly into an “empty flask” which forces the air out into another flask containing concentrated sulphuric (VI) acid. Concentrated sulphuric (VI) acid is hygroscopic. It therefore absorb/remove water present in the air sample.
More water forces the air into the flask containing either concentrated sodium hydroxide or potassium hydroxide solution. These alkalis react with carbon IV) oxide to form the carbonates and thus absorbs/remove carbon IV) oxide present in the air sample.
Chemical equation 2NaOH (aq) + CO2 (g) -> Na2CO3 (aq) + H2O(l)
Chemical equation 2KOH (aq) + CO2 (g) -> K2CO3 (aq) + H2O(l)
More water forces the air through a glass tube packed with copper turnings. Heated brown copper turnings react with oxygen to form black copper (II) oxide.
Chemical equation 2Cu (s) + O2 (g) -> CuO (s)
(brown) (black)
The remaining gas mixture is collected by upward delivery/downward displacement of water/over water. It contains about 99% nitrogen and 1% noble gases.
On a large scale for industrial purposes, nitrogen is got from fractional distillation of air.
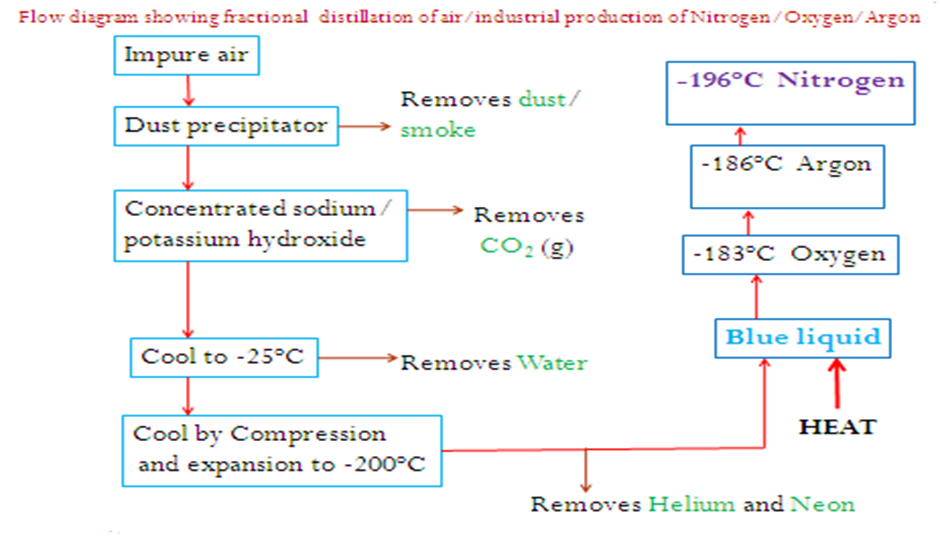
c) Nitrogen from fractional distillation of air.
For commercial purposes nitrogen is got from the fractional of air.
Air is first passed through a dust precipitator/filter to remove dust particles.
The air is then bubbled through either concentrated sodium hydroxide or potassium hydroxide solution to remove/absorb Carbon(IV) oxide gas.
Chemical equation 2NaOH (aq) + CO2 (g) -> Na2CO3 (aq) + H2O(l)
Chemical equation 2KOH (aq) + CO2 (g) -> K2CO3 (aq) + H2O(l)
Air mixture is the cooled to -25oC.At this temperature, water (vapour ) liquidifies and then solidify to ice and thus removed.
The air is further cooled to -200oC during which it forms a blue liquid.
The liquid is then heated. Nitrogen with a boiling point of -196oC distils first then Argon at-186oC and then finally Oxygen at -183oC boils last.
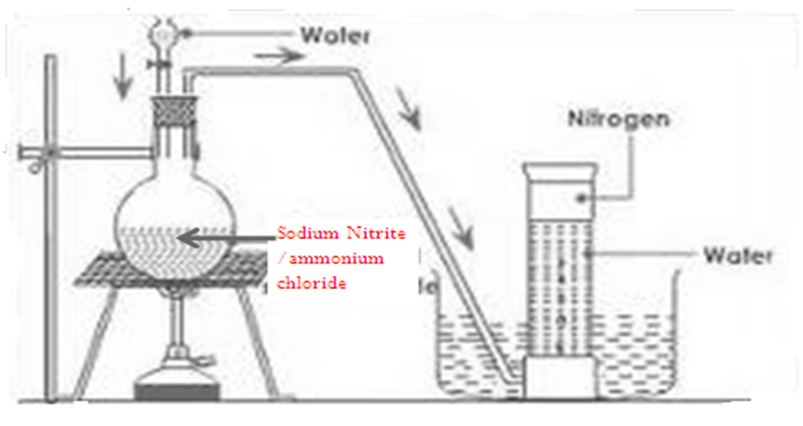
d) School laboratory preparation of Nitrogen.
The diagram below shows the set up of the school laboratory preparation of nitrogen gas.
d.Properties of Nitrogen gas(Questions)
1.Write the equation for the reaction for the school laboratory preparation of nitrogen gas.
Chemical equation NH4Cl (s) + NaNO2(s)->NaCl (g)+ NH4NO2 (s)
Chemical equation NH4NO2 (s) -> N2 (g) + H2O (l)
2. State three physical properties of nitrogen gas.
– colourless, odourless, less dense than air ,neutral and slightly soluble in water
3. State and explain the observation made when a burning magnesium ribbon is lowered in a gas jar containing nitrogen gas.
Observation; It continues burning with a blight blindening flame forming white ash.
Explanation
Magnesium burns to produce enough heat /energy to reacts with nitrogen to form white magnesium nitride.
Chemical equation3Mg (s) + N2 (g) -> Mg3N2 (s)
(white ash/solid)
4. State two main uses of nitrogen gas
-manufacture of ammonia from Haber process
– As a refrigerant in storage of semen for Artificial insemination.
B. OXIDES OF NITROGEN
Nitrogen forms three main oxides:
i)Nitrogen(I) oxide(N2O)
ii) Nitrogen(II) oxide (NO)
iii) Nitrogen (IV) oxide( NO2)
i) Nitrogen (I) oxide(N2O)
a) Occurrence
Nitrogen (I) oxide does not occur naturally but prepared in a laboratory.
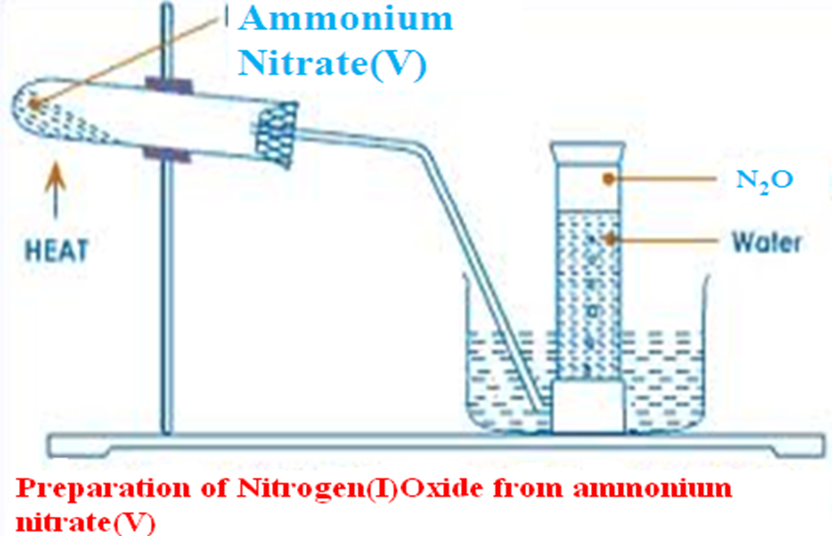
b)Preparation
The set up below shows the set up of apparatus that can be used to prepare Nitrogen (I) oxide in a school laboratory.
c) Properties of nitrogen (I) oxide (Questions)
1. Write the equation for the reaction for the school laboratory preparation of Nitrogen (I) oxide.
Chemical equation NH4NO2(s) -> H2O (l) + N2O (g)
2.a) State and explain three errors made in the above set up
–Oxygen is being generated instead of Nitrogen (I) oxide.
Ammonium Nitrate(V) should be used instead of potassium manganate(VI) and manganese(IV)oxide.
b) State three physical properties of Nitrogen (I) oxide.
-slightly soluble in water.
-colourless
-odourless
-less dense than air
-slightly sweet smell
3. State and explain the observation made when a burning magnesium ribbon is lowered in a gas jar containing Nitrogen (I) oxide.
Observation – Continues to burn with a bright flame
-White solid/residue is formed
Explanation-Magnesium burns in air to produce enough heat/energy split/break Nitrogen (I) oxide gas into free Nitrogen and oxygen then continues to burn in oxygen to form white solid/ash of Magnesium oxide.
Chemical equation
Mg(s) + N2O (g)-> MgO (s) + N2(g)
4. State and explain the observation made when the following non metals are burnt then lowered in a gas jar containing Nitrogen (I) oxide.
a) Carbon/charcoal
Observation – Continues to burn with an orange glow
-colorless gas is formed that forms white precipitate with lime water.
Explanation-Carbon/charcoal burns in air to produce enough heat/energy split/break Nitrogen (I) oxide gas into free Nitrogen and oxygen then continues to burn in oxygen to form carbon (IV) oxide gas. Carbon (IV) oxide gas reacts to form a white precipitate with lime water.
Chemical equation C(s) + 2N2O (g)-> CO2 (g) + 2N2(g)
b) Sulphur powder
Observation – Continues to burn with a blue flame
-colorless gas is formed that turn orange acidified potassium dichromate (VI) to green.
Explanation-Sulphur burns in air to produce enough heat/energy split/break Nitrogen (I) oxide gas into free Nitrogen and oxygen then continues to burn in oxygen to form sulphur (IV) oxide gas. Sulphur (IV) oxide gas turns orange acidified potassium dichromate (VI) to green.
Chemical equation S(s) + 2N2O (g)-> SO2 (g) + 2N2(g)
5. State two uses of nitrogen (I) oxide
-As laughing gas because as anesthesia the patient regain consciousness laughing hysterically after surgery.
-improves engine efficiency.
6. State three differences between nitrogen (I) oxide and oxygen
–Oxygen is odourless while nitrogen (I) oxide has faint sweet smell
-Both relight/rekindle a glowing wooden splint but Oxygen can relight a feeble glowing splint while nitrogen (I) oxide relights well lit splint.
-Both are slightly soluble in water but nitrogen (I) oxide is more soluble.
ii) Nitrogen (II) oxide (NO)
a) Occurrence
Nitrogen (II) oxide does not occur naturally but prepared in a laboratory.
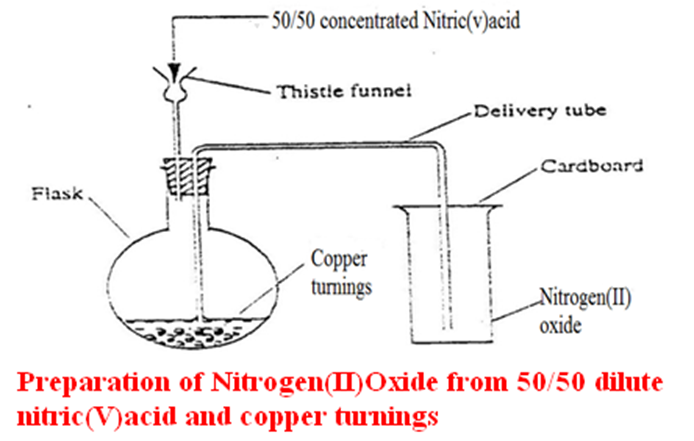
b)Preparation
The set up below shows the set up of apparatus that can be used to prepare Nitrogen (II) oxide in a school laboratory.
c) Properties of nitrogen (II) oxide (Questions)
- Write the equation for the reaction for the school laboratory preparation of Nitrogen (II) oxide.
Chemical equation 3Cu(s) + 8HNO3(aq) -> 4H2O (l)+2NO (g) +2Cu(NO3)2(aq)
Chemical equation 3Zn(s) + 8HNO3(aq) -> 4H2O (l)+2NO (g) +2Zn(NO3)2(aq)
Chemical equation 3Mg(s) + 8HNO3(aq) -> 4H2O (l)+2NO (g)+2Mg(NO3)2(aq)
2. State three physical properties of Nitrogen (II) oxide.
-insoluble in water.
-colourless
-odourless
-denser dense than air
-has no effect on both blue and red litmus papers
- State and explain the observation made when a burning magnesium ribbon is lowered in a gas jar containing Nitrogen (II) oxide.
Observation – Continues to burn with a bright flame
-White solid/residue is formed
Explanation-Magnesium burns in air to produce enough heat/energy split/break Nitrogen (II) oxide gas into free Nitrogen and oxygen then continues to burn in oxygen to form white solid/ash of Magnesium oxide.
Chemical equation 2Mg(s) + 2NO (g)-> 2MgO (s) + N2(g)
- State and explain the observation made when the following non metals are burnt then lowered in a gas jar containing Nitrogen (II) oxide.
a) Carbon/charcoal
Observation – Continues to burn with an orange glow
-colorless gas is formed that forms white precipitate with lime water.
Explanation-Carbon/charcoal burns in air to produce enough heat/energy split/break Nitrogen (II) oxide gas into free Nitrogen and oxygen then continues to burn in oxygen to form carbon (IV) oxide gas.Carbon (IV) oxide gas reacts to form a white precipitate with lime water.
Chemical equation C(s) + 2NO (g)-> CO2 (g) + N2(g)
b) Sulphur powder
Observation – Continues to burn with a blue flame
-colorless gas is formed that turn orange acidified potassium dichromate (VI) to green.
Explanation-Sulphur burns in air to produce enough heat/energy split/break Nitrogen (II) oxide gas into free Nitrogen and oxygen then continues to burn in oxygen to form sulphur (IV) oxide gas.Sulphur (IV) oxide gas turns orange acidified potassium dichromate (VI) to green.
Chemical equation S(s) + N2O (g)-> SO2 (g) + N2(g)
c) Phosphorus
Observation – Continues to produce dense white fumes
Explanation-Phosphorus burns in air to produce enough heat/energy split/break Nitrogen (II) oxide gas into free Nitrogen and oxygen then continues to burn in oxygen to form dense white fumes of phosphorus (V) oxide gas.
Chemical equation 4P(s) + 10NO (g)-> 2P2O5(g) + 5N2(g)
5. State one use of nitrogen (II) oxide
As an intermediate gas in the Ostwalds process for manufacture of nitric(V) gas.
6. State and explain the observation made when nitrogen (II) oxide is exposed to the atmosphere.
Observation–brown fumes produced/evolved that turn blue litmus paper red.
Explanation- Nitrogen (II) oxide gas on exposure to air is quickly oxidized by the air/ oxygen to brown nitrogen (IV) oxide gas. Nitrogen (IV) oxide gas is an acidic gas.
Chemical equation 2NO (g)+ O2(g)-> 2NO2 (g)
(colorless) (brown)
ii) Nitrogen (IV) oxide (NO2)
a) Occurrence
Nitrogen (IV) oxide occurs -naturally from active volcanic areas.
-formed from incomplete combustion of the internal combustion engine of motor vehicle exhaust fumes.
-from lightening
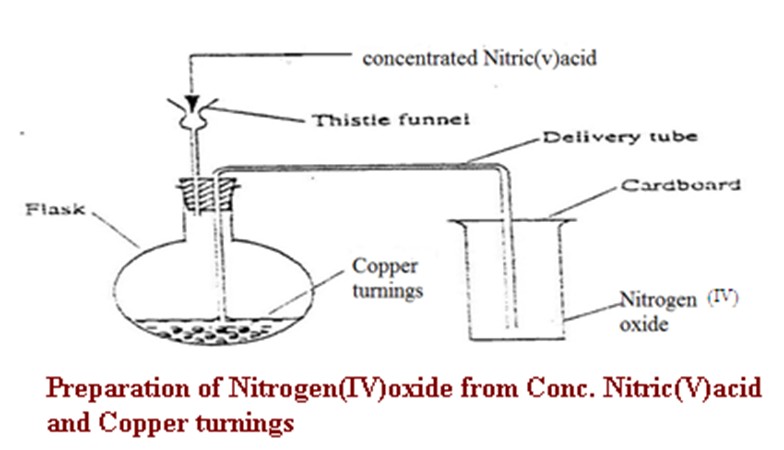
b)Preparation
The set up below shows the set up of apparatus that can be used to prepare Nitrogen (IV) oxide in a school laboratory.
c) Properties of nitrogen (IV)oxide (Questions)
1. Write the equation for the reaction for the school laboratory preparation of Nitrogen (II) oxide.
Chemical equation Cu(s) + 4HNO3(aq) -> 2H2O (l)+2NO 2(g) +Cu(NO3)2(aq)
Chemical equation Zn(s) + 4HNO3(aq) -> 2H2O (l)+2NO 2(g) +Zn(NO3)2(aq)
Chemical equation Fe(s) + 4HNO3(aq) -> 2H2O (l)+2NO 2(g) +Fe(NO3)2(aq)
2. State three physical properties of Nitrogen (IV) oxide.
-soluble/dissolves in water.
-brown in colour
-has pungent irritating poisonous odour/smell
-denser dense than air
-turns blue litmus papers to red
3. State and explain the observation made when Nitrogen (IV) oxidegas is bubbled in water.
Observation–The gas dissolves and thus brown colour of the gas fades
-A colourless solution is formed
-solution formed turns blue litmus papers to red
-solution formed has no effect on red
Explanation-Magnesium burns in air to produce enough heat/energy split/break Nitrogen (IV) oxide gas dissolves then react with water to form an acidic mixture of nitric(V) acid andnitric(III) acid.
Chemical equationH2O (l) + 2NO 2(g)->HNO3(aq) + HNO2(aq)
(nitric(V) acid) (nitric(III) acid)
4. State and explain the observation made when a test tube containing Nitrogen (IV) oxide is cooled then heated gently then strongly.
Observation on cooling
-Brown colour fades
-Yellow liquid formed
Observation on gentle heating
–Brown colour reappears
–Yellow liquid formed changes to brown fumes/gas
Observation on gentle heating
–Brown colour fades
–brown fumes/gas changes to a colourless gas
Explanation-Brown nitrogen (IV) oxide gas easily liquefies to yellow dinitrogen tetraoxide liquid.When the yellow dinitrogen tetraoxide liquid is gently heated it changes back to the brown nitrogen (IV) oxidegas.When the brown nitrogen (IV) oxide gas is strongly heated it decomposes to colourless mixture of Nitrogen (II) oxide gas and Oxygen.
Chemical equation O2(s) + 2NO (g) ===== 2NO2 (g) ===== N2O4(l)
(colourless gases) (brown gas) (yellow liquid)
5. State and explain the observation made when a burning magnesium ribbon is lowered in a gas jar containing Nitrogen (IV) oxide.
Observation – Continues to burn with a bright flame
-White solid/residue is formed
-Brown fumes/colour fades
Explanation-Magnesium burns in air to produce enough heat/energy split/break brown Nitrogen (IV) oxide gas into free colourless Nitrogen and oxygen then continues to burn in oxygen to form white solid/ash of Magnesium oxide.
Chemical equation 4Mg(s) + 2NO 2(g)-> 4MgO (s) + N2(g)
4. State and explain the observation made when the following non metals are burnt then lowered in a gas jar containing Nitrogen (IV) oxide.
a) Carbon/charcoal
Observation – Continues to burn with an orange glow
-Brown fumes/colour fades
-colorless gas is formed that forms white precipitate with lime water.
Explanation-Carbon/charcoal burns in air to produce enough heat/energy split/break brown Nitrogen (IV) oxide gas into free colourless Nitrogen and oxygen then continues to burn in oxygen to form carbon (IV) oxide gas.Carbon (IV) oxide gas reacts to form a white precipitate with lime water.
Chemical equation2C(s) + 2NO 2(g)-> 2CO2 (g) + N2(g)
b) sulphur powder
Observation – Continues to burn with a blue flame
-Brown fumes/colour fades
-colorless gas is formed that turn orange acidified potassium dichromate (VI) to green.
Explanation-Sulphur burns in air to produce enough heat/energy split/break brown Nitrogen (IV) oxide gas into free colourless Nitrogen and oxygen then continues to burn in oxygen to form sulphur (IV) oxide gas.Sulphur (IV) oxide gas turns orange acidified potassium dichromate (VI) to green.
Chemical equation2S(s) + 2NO2 (g)-> 2SO2 (g) + N2(g)
c) Phosphorus
Observation- Continues to produce dense white fumes
-Brown fumes/colour fades
Explanation-Phosphorus burns in air to produce enough heat/energy split/break brown Nitrogen (IV) oxide gas into free colourless Nitrogen and oxygen then continues to burn in oxygen to form dense white fumes of phosphorus (V) oxide gas.
Chemical equation 8P(s) + 10NO2 (g)-> 4P2O5(g) + 5N2(g)
5. State two uses of nitrogen (IV) oxide
-In theOstwald process for industrial manufacture of nitric (V) gas.
-In the manufacture of T.N.T explosives
6. State and explain the observation made when nitrogen (II) oxide is exposed to the atmosphere.
Observation–brown fumes produced/evolved that turn blue litmus paper red.
Explanation- Nitrogen (II) oxide gas on exposure to air is quickly oxidized by the air/ oxygen to brown nitrogen (IV) oxide gas. Nitrogen (IV) oxide gas is an acidic gas.
Chemical equation 2NO (g) + O2(g) -> 2NO2 (g)
(colourless) (brown)
C. AMMONIA (NH3)
Ammonia is a compound of nitrogen and hydrogen only. It is therefore a hydride of nitrogen.
a) Occurrence
Ammonia gas occurs -naturally from urine of mammals and excretion of birds
-formed in the kidney of human beings
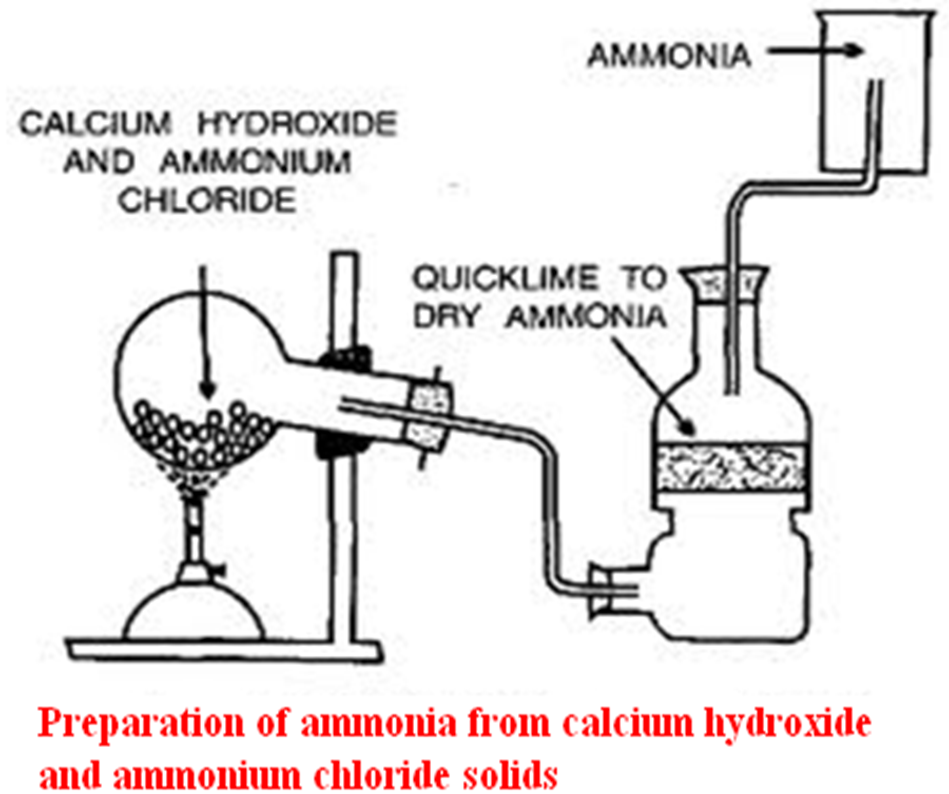
b)Preparation
The set up below shows the set up of apparatus that can be used to prepare dry Ammonia gas in a school laboratory.
Set up method 1
1. Write the equation for the reaction taking place in:
- Method 1
Chemical equation
Ca (OH)2(s) + NH4 Cl(s)->CaCl2 (aq) + H2O(l) + 2NH3(g)
b)Method 2
Chemical equation
NaOH (aq) + NH4 Cl(aq) -> NaCl (aq) + H2O(l) + NH3(g)
2. State three physical properties of ammonia.
-has a pungent choking smell of urine
-Colourless
-Less dense than air hence collected by upward delivery
-Turns blue litmus paper blue thus is the only naturally occurring basic gas (at this level)
3. Calcium oxide is used as the drying agent. Explain why calcium chloride and concentrated sulphuric(VI) acid cannot be used to dry the gas.
-Calcium chloride reacts with ammonia forming the complex compound CaCl2.8H2O.
Chemical equation CaCl2 (s) + 8NH3(g) -> CaCl2 .8NH3(g)
-Concentrated sulphuric(VI) acid reacts with ammonia forming ammonium sulphate(VI) salt compound
Chemical equation 2NH3(g) +H2SO4(l) ->(NH4)2SO4(aq)
4. Describe the test for the presence of ammonia gas.
Using litmus paper:
Dip moist/damp/wet blue and red litmus papers in a gas jar containing a gas suspected to be ammonia.The blue litmus paper remain blue and the red litmus paper turns blue.Ammonia is the only basic gas.(At this level)
Using hydrogen chloride gas
Dip a glass rod in concentrated hydrochloric acid. Bring the glass rod near the mouth of a gas jar suspected to be ammonia. White fumes (of ammonium chloride)are produced/evolved.
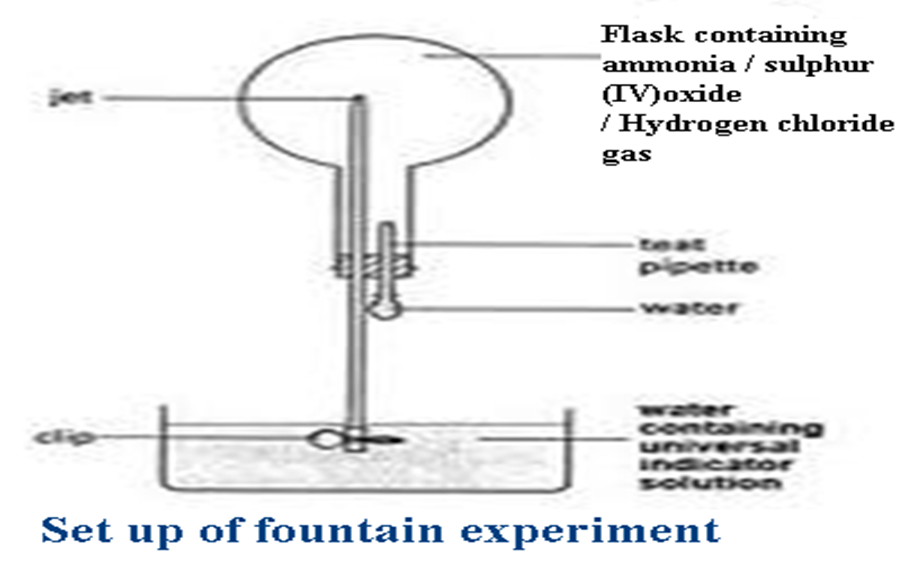
5. Describe the fountain experiment to show the solubility of ammonia.
Ammonia is very soluble in water.
When a drop of water is introduced into flask containing ammonia, it dissolves all the ammonia in the flask. If water is subsequently allowed into the flask through a small inlet, atmospheric pressure forces it very fast to occupy the vacuum forming a fountain. If the water contains three/few drops of litmus solution, the litmus solution turns blue because ammonia is an alkaline/basic gas. If the water contains three/few drops of phenolphthalein indicator, the indicator turns pink because ammonia is an alkaline/basic gas. Sulphur(IV)oxide and hydrogen chloride gas are also capable of the fountain experiment . If the water contains three/few drops of phenolphthalein indicator, the indicator turns colourless because both Sulphur(IV) oxide and hydrogen chloride gas are acidic gases.
6. State and explain the observation made when hot platinum /nichrome wire is placed over concentrated ammonia solution with Oxygen gas bubbled into the mixture.
Observations
Hot platinum /nichrome wire continues to glow red hot.
Brown fumes of a gas are produced.
Explanation
Ammonia reacts with Oxygen on the surface of the wire .This reaction is exothermic producing a lot of heat/energy that enables platinum wire to glow red hot. Ammonia is oxidized to Nitrogen(II)oxide gas and water. Hot platinum /nichrome wire acts as catalyst to speed up the reaction. Nitrogen(II)oxide gas is further oxidized to brown Nitrogen(IV)oxide gas on exposure to air.
Chemical equation
(i)4NH3(g) + 5O2(g) -Pt-> 4NO(g) + 6H2O(l)
(ii)2NO(g) + O2(g) -> 2NO2(g)
7. Ammonia gas was ignited in air enriched with Oxygen gas. State and explain the observations made
Observations
– Ammonia gas burns with a green flame
-Colourless gas produced
Explanation
Ammonia gas burns with a green flame in air enriched with Oxygen to from Nitrogen gas and water.
Chemical equation
2NH3(g) + O2(g) -> N2(g) + 3H2O(l)
8. Dry ammonia was passed through heated copper(II)Oxide as in the set up below.
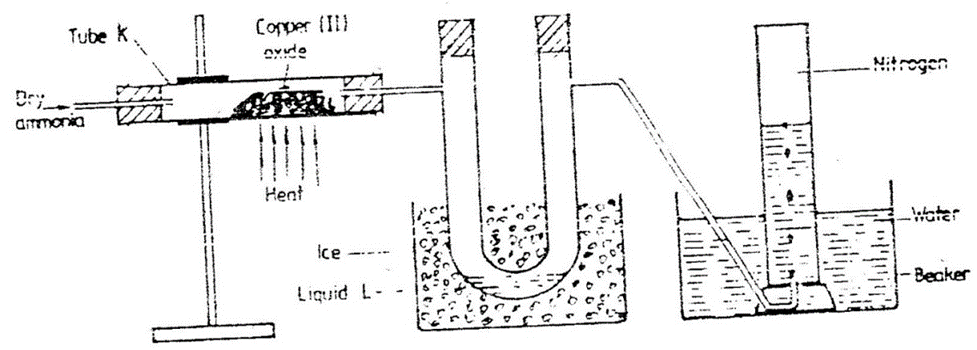
(a)State the observations made in tube K
-Colour changes from black to brown
-Colourless liquid droplet form on the cooler parts of tube K
(b)(i)Identify liquid L.
-Water/ H2O(l)
(ii)Explain a chemical and physical test that can be used to identify liquid L.
Chemical test
(i) Add three/few drops of liquid L into anhydrous copper(II)sulphate(VI).
Colour changes from white to blue.
Explanation-Water changes white anhydrous copper(II)sulphate(VI) to blue hydrated copper(II)sulphate(VI)
(ii) Add three/few drops of liquid L into anhydrous cobalt(II)Chloride.
Colour changes from blue to pink.
Explanation-Water changes blue anhydrous cobalt(II)Chloride to pink hydrated cobalt(II)Chloride.
Physical test
(i)Heat the liquid. It boils at 100oC at sea level (1atmosphere pressure/760mmHg pressure, 101300Pa,101300Nm-2).
(ii)Cool the liquid. It freezes at 0.0oC .
(iii)Determine the density. It is 1.0gcm-3
(c)Write the equation for the reaction that take place.
2NH3(g) + 3CuO(s) -> N2(g) + 3H2O(l) + 3Cu(s)
(black) (brown)
2NH3(g) + 3PbO(s) -> N2(g) + 3H2O(l) + 3Pb(s)
(brown when hot) (grey)
8.(a)What is aqueous ammonia
Aqueous ammonia is formed when ammonia gas is dissolved in water.
NH3(g) + (aq) -> NH3(aq)
A little NH3(aq) reacts with ammonia water to form ammonia solution(NH4OH)
NH3 (aq) + H2O(l) OH–(aq) + NH4+(aq)
This makes a solution of aqueous ammonia is a weak base /alkali unlike other two alkalis.
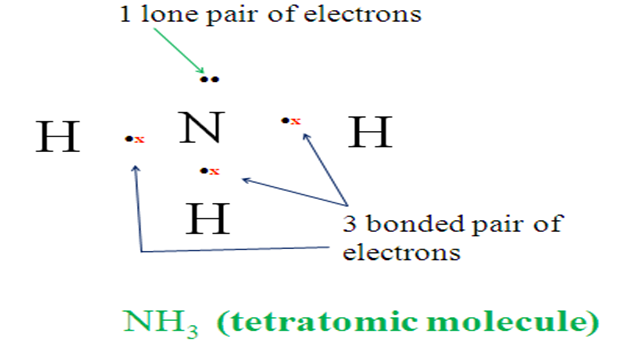
9.Using dot and cross to represent outer electrons show the bonding in:
(a) NH3
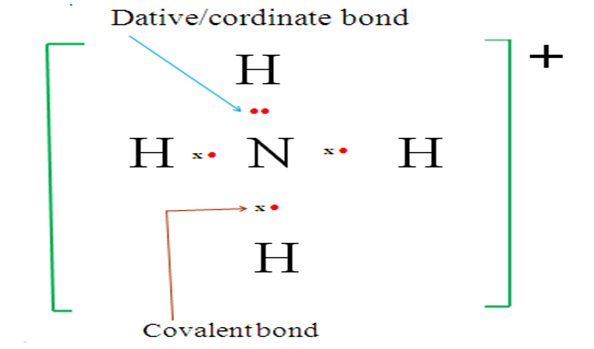
(b) NH4+
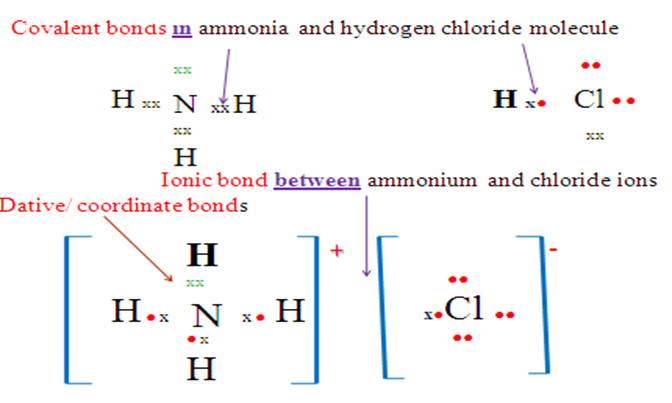
(c)NH4Cl
10.Name four uses of ammonia
(i)In the manufacture of nitrogenous fertilizers.
(ii) In the manufacture of nitric(V)acid from Ostwalds process.
(iii)As a refrigerant in ships and warehouses.
(iv)In softening hard water.
(v)In the solvay process for the manufacture of sodium carbonate.
(vi)In the removal of grease and stains.
11.(a)Calculate the percentage of Nitrogen in the following fertilizers:
(i) (NH4)2SO4
Molar mass of (NH4)2SO4 = 132g
Mass of N in (NH4)2SO4= 28g
% of N => 28 x 100 = 21.2121%
132
(ii) (NH4)3PO4
Molar mass of (NH4)3PO4 = 149g
Mass of N in (NH4)3PO4= 42g
% of N => 42 x 100 = 28.1879%
149
(b)State two advantages of fertilizer a (i) over a (ii) above.
(i)Has higher % of Nitrogen
(ii)Has phosphorus which is necessary for plant growth.
(c) Calculate the mass of Nitrogen in a 50kg bag of:
(i) (NH4)2SO4
% of N in (NH4)2SO4 = 21.2121%
Mass of N in 50 kg (NH4)2SO4= 21.2121 x 50 = 10.6 kg
100
(ii) NH4NO3
Molar mass of NH4NO3 = 80g
Mass of N in (NH4)3PO4= 28g
% of N => 28 x 100 = 35%
80
% of N in NH4NO3 = 35%
Mass of N in 50 kg (NH4)2SO4= 35 x 50 = 17.5 kg
100
NH4NO3 therefore has a higher mass of Nitrogen than (NH4)2SO4
d).Manufacture of Ammonia /Haber process
Most of the Ammonia produced for industrial purposes uses the Haber process developed by the German Scientist Fitz Haber.
(i)Raw materials
The raw materials include:
(i)Nitrogen from Fractional distillation of air from the atmosphere.
(ii)Hydrogen from:
I. Water gas-passing steam through heated charcoal
C(s) + H2O(l) -> CO(g) + H2 (g)
II .Passing natural gas /methane through steam.
CH4(g)+ H2O(l) -> CO(g) + 3H2 (g)
(ii)Chemical process
Hydrogen and Nitrogen are passed through a purifier to remove unwanted gases like Carbon(IV)oxide,Oxygen,sulphur(IV)oxide, dust, smoke which would poison the catalyst.
Hydrogen and Nitrogen are then mixed in the ratio of 3:1 respectively. The mixture is compressed to 200-250atmoshere pressure to liquidify. The liquid mixture is then heated to 400- 450oC.The hot compressed gases are then passed over finely divided Iron catalyst promoted/impregnated with Al2O3 /K2O .Promoters increase the efficiency of the catalyst.
