A.SULPHUR (S)
Sulphur is an element in Group VI Group 16)of the Periodic table . It has atomic number 16 and electronic configuration 16 and valency 2 /divalent and thus forms the ion S2-
A. Occurrence.
Sulphur mainly occurs:
(i) as free element in Texas and Louisiana in USA and Sicily in Italy.
(ii)Hydrogen sulphide gas in active volcanic areas e.g. Olkaria near Naivasha in Kenya
(iii)as copper pyrites(CuFeS2) ,Galena (PbS,Zinc blende(ZnS))and iron pyrites(FeS2) in other parts of the world.
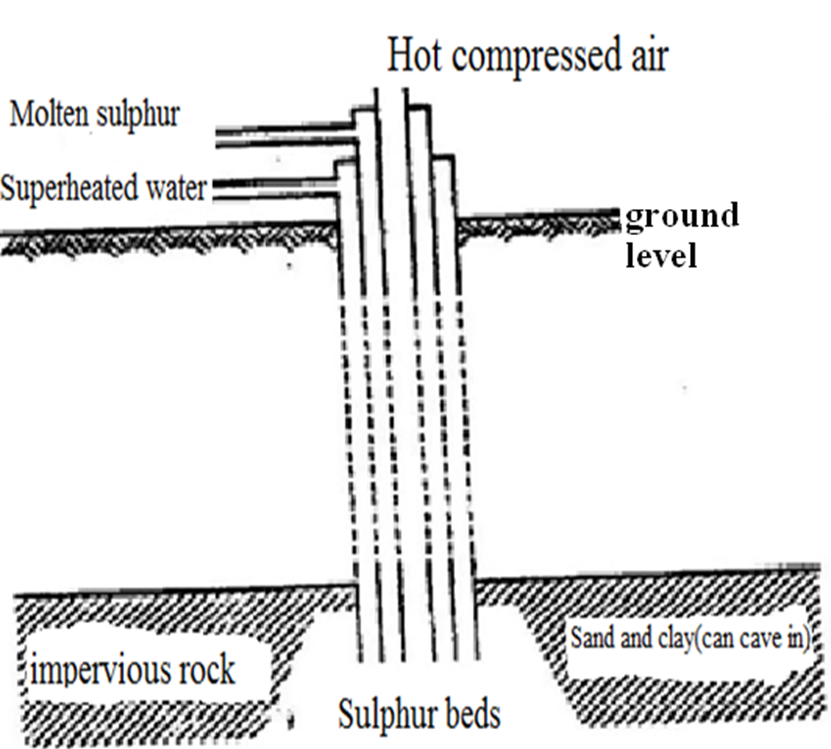
B. Extraction of Sulphur from Fraschs process
Suphur occurs about 200 metres underground. The soil structure in these areas is usually weak and can easily cave in.
Digging of tunnels is thus discouraged in trying to extract the mineral.
Sulphur is extracted by drilling three concentric /round pipes of diameter of ratios 2:8: 18 centimeters.
Superheated water at 170oC and 10atmosphere pressure is forced through the outermost pipe.
The high pressures ensure the water remains as liquid at high temperatures instead of vapour of vapour /gas.
The superheated water melts the sulphur because the melting point of sulphur is lower at about at about 115oC.
A compressed air at 15 atmospheres is forced /pumped through the innermost pipe.
The hot air forces the molten sulphur up the middle pipe where it is collected and solidifies in a large tank.
It is about 99% pure.
Diagram showing extraction of Sulphur from Fraschs Process
C. Chemistry: Allotropes of Sulphur.
1. Sulphur exists as two crystalline allotropic forms:
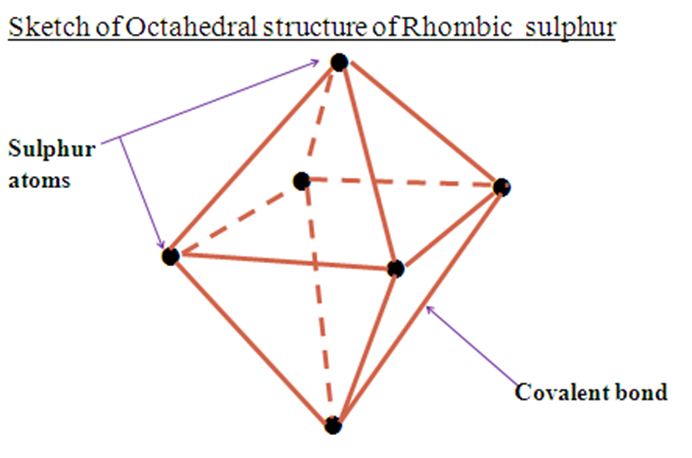
(i)Rhombic sulphur
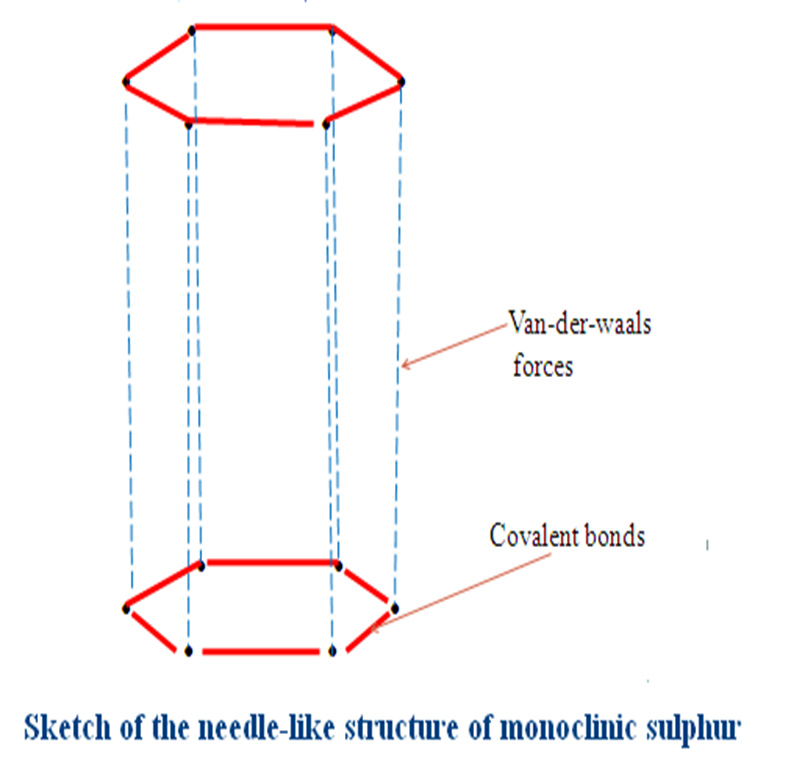
(ii)Monoclinic sulphur
| Rhombic sulphur | Monoclinic sulphur |
| Bright yellow crystalline solid Has a melting point of 113oC Has a density of 2.06gcm-3 Stable below 96oC Has octahedral structure | Pale yellow crystalline solid Has a melting point of 119oC Has a density of 1.96gcm-3 Stable above 96oC Has a needle-like structure |
Rhombic sulphur and Monoclinic sulphur have a transition temperature of 96oC.This is the temperature at which one allotrope changes to the other.
2. Sulphur exists in non-crystalline forms as:
(i)Plastic sulphur-
Plastic sulphur is prepared from heating powdered sulphur to boil then pouring a thin continuous stream in a beaker with cold water. A long thin elastic yellow thread of plastic sulphur is formed .If left for long it turn to bright yellow crystalline rhombic sulphur.
(ii)Colloidal sulphur-
Colloidal sulphur is formed when sodium thiosulphate (Na2S2O3) is added hydrochloric acid to form a yellow precipitate.
D. Heating Sulphur.
A molecule of sulphur exists as puckered ring of eight atoms joined by covalent bonds as S8.
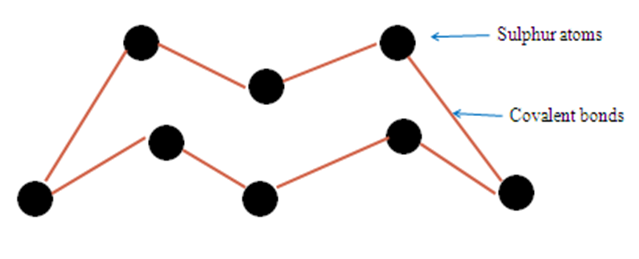
On heating the yellow sulphur powder melts at 113oC to clear amber liquid with low viscosity and thus flows easily.
On further heating to 160oC the molten liquid darkens to a brown very viscous liquid that does not flow easily.
This is because the S8 rings break into S8 chain that join together to form very long chains made of over 100000 atoms of Sulphur.
The long chains entangle each other reducing their mobility /flow and hence increases their viscosity.
On continued further heating to above 160oC, the viscous liquid darkens but becomes more mobile/flows easily and thus less viscous.
This is because the long chains break to smaller/shorter chains.
At 444oC, the liquid boils and forms brown vapour of a mixture of S8 ,S6 ,S2 molecules that solidifies to S8 ring of “flowers of sulphur” on the cooler parts.
Summary of changes on heating sulphur
| Observation on heating | Explanation/structure of Sulphur |
| Solid sulphur Heat to 113oC Amber yellow liquid Heat to 160oC Liquid darkens Heat to 444oC Liquid boils to brown vapour Cool to room temperature Yellow sublimate (Flowers of Sulphur) | Puckered S8 ring Puckered S8 ring in liquid form (low viscosity/flow easily) Puckered S8 ring break/opens then join to form long chains that entangle (very high viscosity/very low rate of flow) Mixture of S8 ,S6 ,S2 vapour Puckered S8 ring |
E. Physical and Chemical properties of Sulphur.(Questions)
1. State three physical properties unique to Sulphur
Sulphur is a yellow solid, insoluble in water, soluble in carbon disulphide/tetrachloromethane/benzene, poor conductor of heat and electricity. It has a melting point of 115oC and a boiling point of 444oC.
2. Moist/damp/wet blue and red litmus papers were put in a gas jar containing air/oxygen. Burning sulphur was then lowered into the gas jar. State and explain the observation made.
Observations
-Sulphur melts then burns with a blue flame
Colourless gas produced that has a pungent smell
Red litmus paper remains red. Blue litmus paper turns red.
Explanation
Sulphur burns in air and faster in Oxygen to form Sulphur(IV)Oxide gas and traces/small amount of Sulphur(VI)Oxide gas. Both oxides react with water to form the corresponding acidic solution i.e
(i) Sulphur(IV)Oxide gas reacts with water to form sulphuric(IV)acid
(ii) Sulphur(VI)Oxide gas reacts with water to form sulphuric(VI)acid
Chemical equation
S(s) + O2(g) -> SO2(g) (Sulphur(IV)Oxide gas)
2S(s) + 3O2(g) -> 2SO3(g) (Sulphur(VI)Oxide gas traces)
SO2(g) + H2O(l) -> H2 SO3 (aq) ( sulphuric(IV)acid) SO3(g) + H2O(l) -> H2 SO4 (aq) ( sulphuric(VI)acid).
3. Iron filings were put in a test tube containing powdered sulphur then heated on a Bunsen flame. Stop heating when reaction starts. State and explain the observations made. Test the effects of a magnet on the mixture before and after heating. Explain.
Observations
Before heating, the magnet attracts iron filings leaving sulphur
After heating, the magnet does not attract the mixture.
After heating, a red glow is observed that continues even when heating is stopped..
Black solid is formed.
Explanation Iron is attracted to a magnet because it is ferromagnetic.
When a mixture of iron and sulphur is heated, the reaction is exothermic giving out heat energy that makes the mixture to continue glowing even after stopping heating.
Black Iron(II)sulphide is formed which is a compound and thus not ferromagnetic.
Chemical equation Fe(s) + S(s) -> FeS(s) (Exothermic reaction/ –∆H)
Heated powdered heavy metals combine with sulphur to form black sulphides.
Cu(s) + S(s) -> CuS(s)
Zn(s) + S(s) -> ZnS(s)
Pb(s) + S(s) -> PbS(s)
4.The set up below show the reaction of sulphur on heated concentrated sulphuric(VI)acid.

(i)State and explain the observation made.
Observation
Yellow colour of sulphur fades
Orange colour of potassium dichromate(VI)paper turns to green.
Explanation
Hot concentrated sulphuric(VI)acid oxidizes sulphur to sulphur (IV)oxide gas. The oxide is also reduced to water. Traces of sulphur (VI)oxide is formed.
Chemical equation
S(s) + 3H2 SO4 (l) -> 3SO2(g) + 3H2O(l) +SO3(g)
Sulphur (IV)oxide gas turns Orange potassium dichromate(VI)paper to green.
(ii)State and explain the observation made if concentrated sulphuric (VI) acid is replaced with concentrated Nitric (V) acid in the above set up.
Observation
Yellow colour of sulphur fades
Colurless solution formed
Brown fumes/gas produced.
Explanation
Hot concentrated Nitric(V)acid oxidizes sulphur to sulphuric (VI)acid. The Nitric (V) acid is reduced to brown nitrogen(IV)oxide gas.
Chemical equation
S(s) + 6HNO3 (l) -> 6NO2(g) + 2H2O(l) +H2SO4 (l)
NB:
Hydrochloric acid is a weaker oxidizing agent and thus cannot oxidize sulphur like the other mineral acids.
5. State three main uses of sulphur .
Sulphur is mainly used in:
(i)Contact process for the manufacture/industrial/large scale production of concentrated sulphuric(VI)acid.
(ii)Vulcanization of rubber to make it harder, tougher, stronger, and more durable.
(iii)Making gun powder and match stick heads
(iv) As ointments to treat fungal infections
6. Revision Practice
The diagram below represents the extraction of sulphur by Fraschs process. Use it to answer the questions that follow.
| N |
| M |
| L |
(a)Name the substances that passes through:
M Superheated water at 170oC and 10 atmosphere pressure
L Hot compressed air
N Molten sulphur
(b)What is the purpose of the substance that passes through L and M?
M- Superheated water at 170oC and 10 atmosphere pressure is used to melt the sulphur
L- Hot compressed air is used to force up the molten sulphur.
(c) The properties of the two main allotropes of sulphur represented by letters A and B are given in the table below. Use it to answer the questions that follow.
| A | B | |
| Appearance | Bright yellow | Pale yellow |
| Density(gcm-3) | 1.93 | 2.08 |
| Melting point(oC) | 119 | 113 |
| Stability | Above 96oC | Below 96oC |
I.What are allotropes?
Different forms of the same element existing at the same temperature and pressure without change of state.
II. Identify allotrope:
- Monoclinic sulphur
B . Rhombic sulphur
III. State two main uses of sulphur.
-Manufacture of sulphuric(VI)acid
-as fungicide
-in vulcanization of rubber to make it harder/tougher/ stronger
-manufacture of dyes /fibres
(d)Calculate the volume of sulphur (IV)oxide produced when 0.4 g of sulphur is completely burnt in excess air.(S = 32.0 ,I mole of a gas occupies 24 dm3 at room temperature)
Chemical equation
S(s) + O2(g) -> SO2(g)
Mole ratio S: SO2 = 1:1
Method 1
32.0 g of sulphur -> 24 dm3 of SO2(g)
0.4 g of sulphur -> 0.4 g x 24 dm3 = 0.3 dm3
32.0 g
Method 2
Moles of sulphur used= Mass of sulphur => 0.4 = 0.0125 moles
Molar mass of sulphur 32
Moles of sulphur used = Moles of sulphur(IV)oxide used=>0.0125 moles
Volume of sulphur(IV)oxide used = Moles of sulphur(IV)oxide x volume of one mole of gas =>0.0125 moles x 24 dm3 = 0.3 dm3
B.COMPOUNDS OF SULPHUR
The following are the main compounds of sulphur:
(i) Sulphur(IV)oxide
(ii) Sulphur(VI)oxide .
(iii) Sulphuric(VI)acid
(iv) Hydrogen Sulphide
(v) Sulphate(IV)/SO32- and Sulphate(VI)/ SO42- salts
(i) Sulphur(IV)oxide(SO2)
(a) Occurrence
Sulphur (IV)oxide is found in volcanic areas as a gas or dissolved in water from geysersand hot springs in active volcanic areas of the world e.g. Olkaria and Hells gate near Naivasha in Kenya.
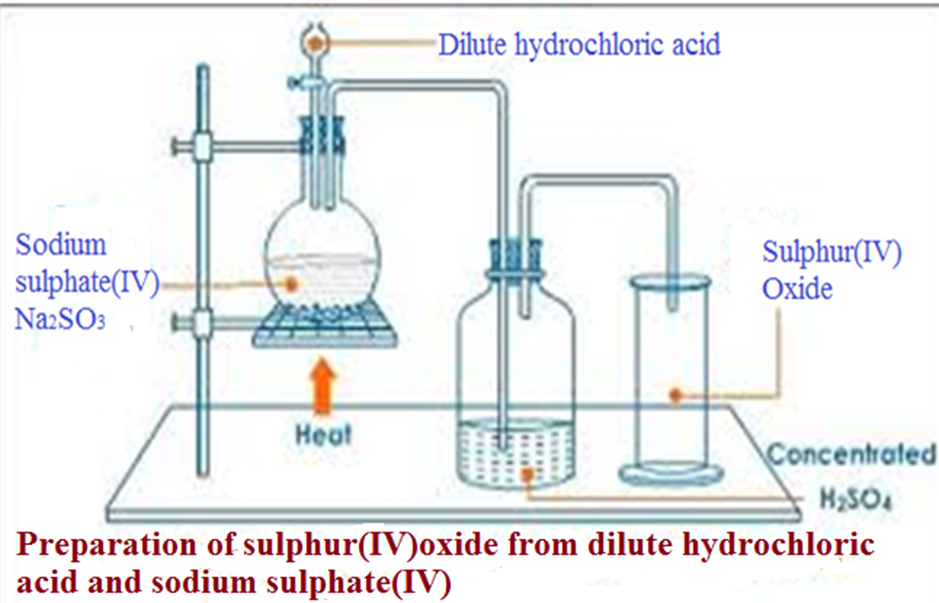
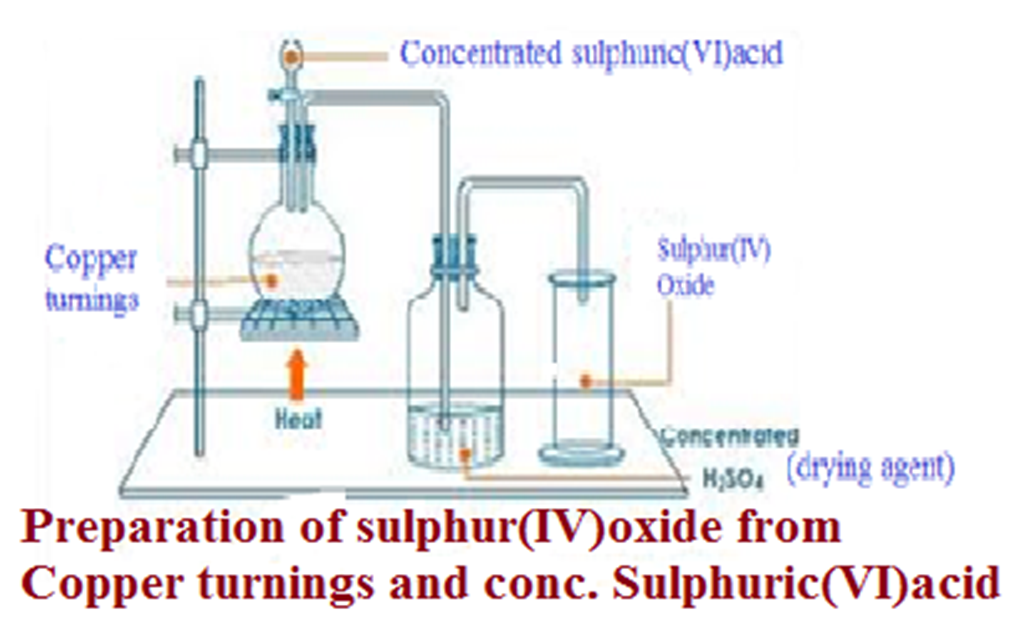
(b) School laboratory preparation
In a Chemistry school laboratory Sulphur (IV)oxide is prepared from the reaction of
Method 1:Using Copper and Sulphuric(VI)acid.
Method 2:Using Sodium Sulphate(IV) and hydrochloric acid.