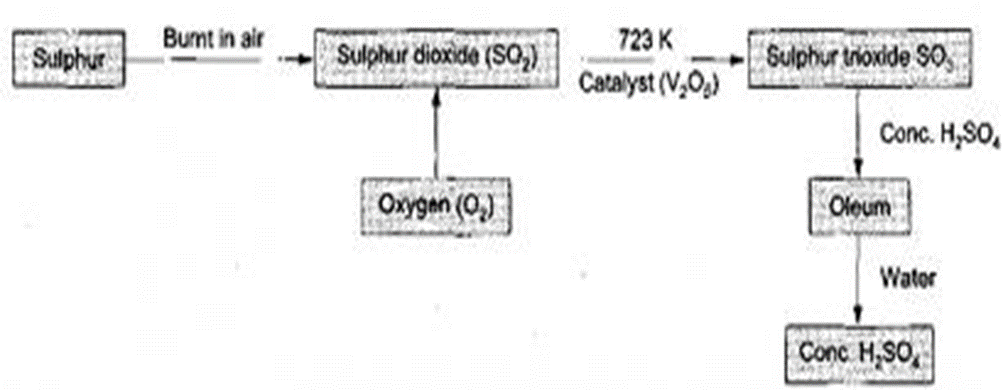
(b)The Contact process for industrial manufacture of H2SO4 .
I. Raw materials
The main raw materials for industrial preparation of Sulphuric(VI)acid include:
(i)Sulphur from Fraschs process or from heating metal sulphide ore like Galena(PbS),Zinc blende(ZnS)
(ii)Oxygen from fractional distillation of air
(iii)Water from rivers/lakes
II. Chemical processes
The contact process involves four main chemical processes:
(i)Production of Sulphur (IV)oxide
As one of the raw materials, Sulphur (IV)oxide gas is got from the following sources;
I. Burning/roasting sulphur in air.
Sulphur from Fraschs process is roasted/burnt in air to form Sulphur (IV)oxide gas in the burners
Chemical equation
S(s) + O2(g) –> SO2 (g)
II. Burning/roasting sulphide ores in air.
Sulphur (IV)oxide gas is produced as a by product in extraction of some metals like:
– Lead from Lead(II)sulphide/Galena,(PbS)
– Zinc from zinc(II)sulphide/Zinc blende, (ZnS)
– Copper from Copper iron sulphide/Copper pyrites, (CuFeS2)
On roasting/burning, large amount /quantity of sulphur(IV)oxide is generated/produced.
Chemical equation
(i)2PbS (s) + 3O2 (g) -> 2PbO(s) + 2SO2 (g)
(ii)2ZnS (s) + 3O2 (g) -> 2ZnO(s) + 2SO2 (g)
(ii)2CuFeS2 (s) + 4O2 (g) -> 2FeO(s) + 3SO2 (g) + Cu2O(s)
Sulphur(IV)oxide easily/readily liquefies and thus can be transported to a far distance safely.
(ii)Purification of Sulphur(IV)oxide
Sulphur(IV)oxide gas contain dust particles and Arsenic(IV)oxide as impurities. These impurities “poison”/impair the catalyst by adhering on/covering its surface.
The impurities are removed by electrostatic precipitation method .
In the contact process Platinum or Vanadium(V)oxide may be used. Vanadium(V)oxide is preferred because it is :
(i) cheaper/less expensive
(ii) less easily poisoned by impurities
(iii)Catalytic conversion of Sulphur(IV)oxide to Sulphur(VI)oxide
Pure and dry mixture of Sulphur (IV)oxide gas and Oxygen is heated to 450oC in a heat exchanger.
The heated mixture is passed through long pipes coated with pellets of Vanadium (V)oxide catalyst.
The close “contact” between the reacting gases and catalyst give the process its name.
Vanadium (V)oxide catalyse the conversion/oxidation of Sulphur(IV)oxide to Sulphur(VI)oxide gas.
Chemical equation
2SO2 (g) + O2(g) — V2O5 –> 2SO2 (g)
This reaction is exothermic (-∆H) and the temperatures need to be maintained at around 450oC to ensure that:
(i)reaction rate/time taken for the formation of Sulphur(VI)oxide is not too slow/long at lower temperatures below 450oC
(ii) Sulphur(VI)oxide gas does not decompose back to Sulphur(IV)oxide gas and Oxygen gas at higher temperatures than 450oC.
(iv)Conversion of Sulphur(VI)oxide of Sulphuric(VI)acid
Sulphur(VI)oxide is the acid anhydride of concentrated Sulphuric(VI)acid. Sulphur(VI)oxide reacts with water to form thick mist of fine droplets of very/highly corrosive concentrated Sulphuric(VI)acid because the reaction is highly exothermic.
To prevent this, Sulphur (VI)oxide is a passed up to meet downward flow of 98% Sulphuric(VI)acid in the absorption chamber/tower.
The reaction forms a very viscous oily liquid called Oleum/fuming Sulphuric (VI) acid/ pyrosulphuric (VI) acid.
Chemical equation
H2SO4 (aq) + SO3 (g) -> H2S2O7 (l)
Oleum/fuming Sulphuric (VI) acid/ pyrosulphuric (VI) acid is diluted carefully with distilled water to give concentrated sulphuric (VI) acid .
Chemical equation
H2S2O7 (l) + H2O(l) -> 2H2SO4 (l)
The acid is stored ready for market/sale.
III. Environmental effects of contact process
Sulphur(VI)oxide and Sulphur(IV)oxide gases are atmospheric pollutants that form acid rain if they escape to the atmosphere.
In the Contact process, about 2% of these gases do not form sulphuric (VI) acid.
The following precautions prevent/minimize pollution from Contact process:
(i)recycling back any unreacted Sulphur(IV)oxide gas back to the heat exchangers.
(ii)dissolving Sulphur(VI)oxide gas in concentrated sulphuric (VI) acid instead of water.
This prevents the formation of fine droplets of the corrosive/ toxic/poisonous fumes of concentrated sulphuric (VI) acid.
(iii)scrubbing-This involves passing the exhaust gases through very tall chimneys lined with quicklime/calcium hydroxide solid.
This reacts with Sulphur (VI)oxide gas forming harmless calcium(II)sulphate (IV) /CaSO3
Chemical equation
Ca(OH)2 (aq) + SO2(g) –> CaSO3 (aq) + H2O (g)
IV. Uses of Sulphuric(VI)acid
Sulphuric (VI) acid is used:
(i) in making dyes and paint
(ii)as acid in Lead-acid accumulator/battery
(iii) for making soapless detergents
(iv) for making sulphate agricultural fertilizers
VI. Sketch chart diagram showing the Contact process