a)Introduction.
Nitric(V)acid is one of the mineral acids .There are three mineral acids; Nitric(V)acid, sulphuric(VI)acid and hydrochloric acid. Mineral acids do not occur naturally but are prepared in a school laboratory and manufactured at industrial level.
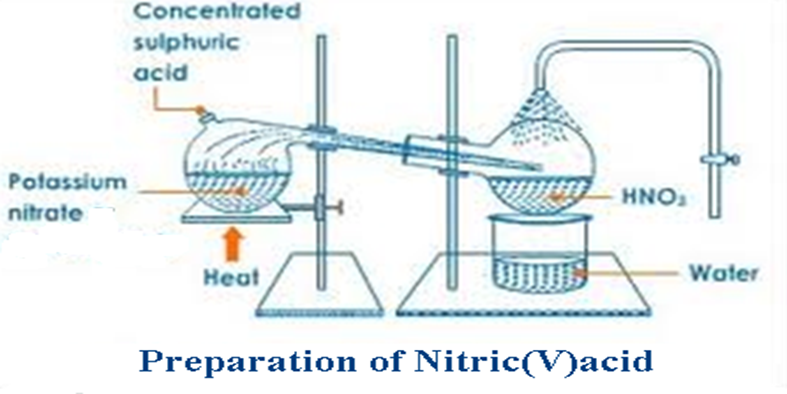
b) School laboratory preparation Nitric(V)acid is prepared in a school laboratory from the reaction of Concentrated sulphuric(VI)acid and potassium nitrate(V) below.
(c)Properties of Concentrated Nitric (V)acid(Questions)
1.Write an equation for the school laboratory preparation of nitric(V)acid.
KNO3(s) + H2SO4(l) -> KHSO4(s) + HNO3(l)
2.Sodium nitrate(V)can also be used to prepare nitric(V)acid. State two reasons why potassium nitrate(V) is preferred over Sodium nitrate(V).
(i) Potassium nitrate(V) is more volatile than sodium nitrate(V) and therefore readily displaced from the less volatile concentrated sulphuric(VI)acid
(ii)Sodium nitrate(V) is hygroscopic and thus absorb water . Concentrated sulphuric(VI)acid dissolves in water. The dissolution is a highly exothermic process.
3. An all glass apparatus /retort is used during the preparation of nitric(V) acid. Explain.
Hotconcentrated nitric(V) acid vapour is highly corrosive and attacks rubber cork apparatus if used.
4. Concentrated nitric(V) acid is colourless . Explain why the prepared sample in the school laboratory appears yellow.
Hot concentrated nitric(V) acid decomposes to brown nitrogen(IV)oxide and Oxygen gases.
4HNO3(l/g) -> 4NO2(g) + H2O (l) +O2(g)
Once formed the brown nitrogen(IV)oxide dissolves in the acid forming a yellow solution .
5. State and explain the observation made when concentrated nitric (V) acid is heated.
Observation
Brown fumes are produced.
Colourless gas that relights/rekindles glowing splint
Explanation
Hot concentrated nitric(V) acid decomposes to water, brown nitrogen(IV)oxide and Oxygen gases. Oxygen gas is not visible in the brown fumes of nitrogen (IV) oxide.
4HNO3(g) -> 4NO2(g) + H2O (l) +O2(g)
6. Explain the observations made when:
(a) About 2cm3 of Iron(II)sulphate(VI) solution is added about 5 drops of concentrated nitric(V) acid and the mixture then heated/warmed in a test tube.
Observation
(i)Colour changes from green to brown.
(ii)brown fumes /gas produced on the upper parts of the test tube.
Explanation
Concentrated nitric(V) acid is a powerful/strong oxidizing agent. It oxidizes green Fe2+ ions in FeSO4 to brown/yellow Fe3+ .The acid is reduced to colourless Nitrogen(II)oxide.
Chemical equation:
6FeSO4(aq) + 3H2SO4 (aq) + 2HNO3(aq) -> 3Fe2(SO4) 3 (aq)+ 4H2O + 2NO(g)
Colourless Nitrogen(II)oxide is rapidly further oxidized to brown Nitrogen(IV)oxide by atmospheric oxygen.
Chemical equation:
2NO(g) + O(g) -> 2NO2 (g)
(colourless) (brown)
(b) A spatula full of sulphur powder in a clean dry beaker was added to 10cm3 concentrated nitric (V) acid and then heated gently/warmed.
Observation
(i)Yellow colour of sulphur fades.
(ii) Brown fumes /gas produced.
Explanation
Concentrated nitric(V) acid is a powerful/strong oxidizing agent. It oxidizes yellow sulphur to colourless concentrated sulphuric(VI)acid. The acid is reduced to brown Nitrogen(IV)oxide gas.
Chemical equation:
S(s) + 6HNO3(l) -> 4NO2(g) + H2O (l) +H2SO4(l)
(c) A few/about 1.0g pieces of copper turnings/Zinc granules/ Magnesium ribbon are added 10cm3 of concentrated nitric(V) acid in a beaker.
Observation
(i) Brown fumes /gas produced.
(ii) Blue solution formed with copper turnings
(iii) Colourless solution formed with Zinc granules/Magnesium ribbon
Explanation
Concentrated nitric (V) acid is a powerful/strong oxidizing agent. It oxidizes metals to their metal nitrate (VI) salts. The acid is reduced to brown Nitrogen (IV) oxide gas.
Chemical equation:
Cu(s) + 4HNO3(l) -> 2NO2(g) + H2O (l) + Cu(NO3) 2 (aq)
Zn(s) + 4HNO3(l) -> 2NO2(g) + H2O (l) + Zn(NO3) 2 (aq)
Mg(s) + 4HNO3(l) -> 2NO2(g) + H2O (l) + Mg(NO3) 2 (aq)
Pb(s) + 4HNO3(l) -> 2NO2(g) + H2O (l) + Pb(NO3) 2 (aq)
Ag(s) + 2HNO3(l) -> NO2(g) + H2O (l) + AgNO3 (aq)
(d)Properties of Dilute Nitric (V)acid(Questions)
(i)What is dilute nitric(v)acid
When concentrated nitric(v)acid is added to over half portion of water ,it is relatively said to be dilute. A dilute solution is one which has more solvent/water than solute/acid. The number of moles of the acid are present in a large amount/volume of the solvent.This makes the molarity /number of moles present in one cubic decimeter of the solution to be low e.g. 0.02M.
If more water is added to the acid until the acid is too dilute to be diluted further then an infinite dilute solution if formed.
(ii))1cm length of polished Magnesium ribbon was put is a test tube containing 0.2M dilute nitric(v)acid. State and explain the observation made.
Observation
-Effervescence/bubbling/fizzing
-Colourless gas produced that extinguish burning splint with an explosion/pop sound
-Colourless solution formed
-Magnesium ribbon dissolves/decrease in size
Explanation
Dilute dilute nitric(v)acid reacts with Magnesium to form hydrogen gas.
Mg(s) + 2HNO3(aq) -> H2 (g) + Mg(NO3) 2 (aq)
With other reactive heavy metals, the hydrogen gas produced is rapidly oxidized to water.
Chemical equation 3Pb(s) + 8HNO3(aq) -> 4H2O (l)+2NO (g) +2Pb(NO3)2(aq)
Chemical equation 3Zn(s) + 8HNO3(aq) -> 4H2O (l)+2NO (g) +2Zn(NO3)2(aq)
Chemical equation 3Fe(s) + 8HNO3(aq) -> 4H2O (l)+2NO (g) +2Fe(NO3)2(aq)
Hydrogen gas therefore is usually not prepared in a school laboratory using dilute nitric (v)acid.
(iii)A half spatula full of sodium hydrogen carbonate and Copper(II) carbonate were separately to separate test tubes containing 10cm3 of 0.2M dilute nitric (V) acid.
Observation
-Effervescence/bubbling/fizzing
-Colourless gas produced that forms a white precipitate with lime water.
-Colourless solution formed withsodium hydrogen carbonate.
– Blue solution formed withCopper(II) carbonate.
Explanation
Dilute dilute nitric (v)acid reacts with Carbonates and hydrogen carbonates to form Carbon(IV)oxide, water and nitrate(V)salt
CuCO3 (s) + 2HNO3(aq) -> H2O (l) + Cu(NO3) 2 (aq) + CO2 (g)
ZnCO3 (s) + 2HNO3(aq) -> H2O (l) + Zn(NO3) 2 (aq) + CO2 (g)
CaCO3 (s) + 2HNO3(aq) -> H2O (l) + Ca(NO3) 2 (aq) + CO2 (g)
PbCO3 (s) + 2HNO3(aq) -> H2O (l) + Pb(NO3) 2 (aq) + CO2 (g)
FeCO3 (s) + 2HNO3(aq) -> H2O (l) + Fe(NO3) 2 (aq) + CO2 (g)
NaHCO3 (s) + HNO3(aq) -> H2O (l) + NaNO3 (aq) + CO2 (g)
KHCO3 (s) + HNO3(aq) -> H2O (l) + KNO3 (aq) + CO2 (g)
NH4HCO3 (aq) + HNO3(aq) -> H2O (l) + NH4NO3 (aq) + CO2 (g)
Ca(HCO3) 2 (aq) + 2HNO3(aq) -> 2H2O (l) + Ca(NO3) 2 (aq) + 2CO2 (g)
Mg(HCO3) 2 (aq) + 2HNO3(aq) -> 2H2O (l) + Mg(NO3) 2 (aq) + 2CO2 (g)
(iii) 25.0cm3 of 0.1M Nitric(V) acid was titrated with excess 0.2M sodium hydroxide solution using phenolphthalein indicator.
I. State the colour change at the end point
Colourless
II. What was the pH of the solution at the end point. Explain.
pH 1/2/3
A little of the acid when added to the base changes the colour of the indicator to show the end point. The end point therefore is acidic with low pH of Nitric(V) acid. Nitric(V) acid is a strong acid with pH 1/2/3.
III. Calculate the number of moles of acid used.
Number of moles = molarity x volume => 0.1 x 25 = 2.5 x 10-3moles
1000 1000
IV. Calculate the volume of sodium hydroxide used
Volume of sodium hydroxide in cm3
= 1000 x Number of moles => 1000x 2.5 x 10-3 = 12.5cm3
Molarity 0.2
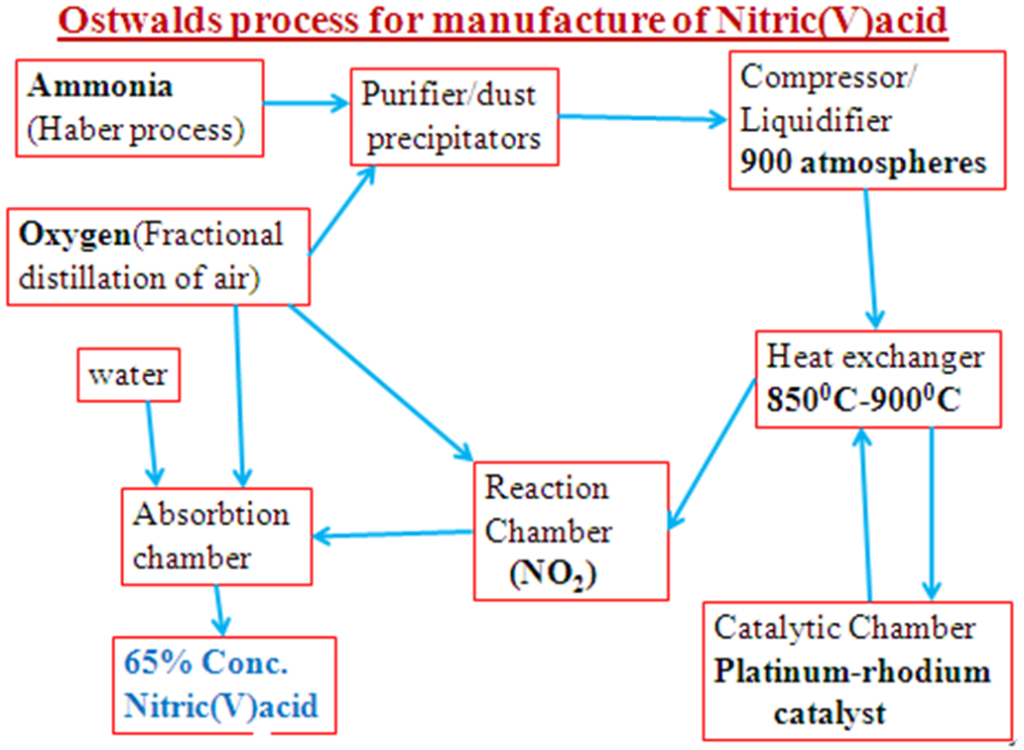
(e)Industrial large scale manufacture of Nitric (V) acid
(i)Raw materials
1. Air/Oxygen
Oxygen is got from fractional distillation of air
Ammonia from Haber process.
2. Chemical processes
Air from the atmosphere is passes through electrostatic precipitators/filters to remove unwanted gases like Nitrogen, Carbon (IV) oxide, dust, smoke which may poison the catalyst. The ammonia -air mixture is compressed to 9 atmospheres to reduce the distance between reacting gases.
The mixture is passed through the heat exchangers where a temperature of 850oC-900oC is maintained.
The first reaction takes place in the catalytic chamber where Ammonia reacts with the air to form Nitrogen (II) Oxide and water.
Optimum condition in Ostwald’s process
Chemical equation
4NH3 (g) + 5O2 (g) === Pt/Rh === 4NO (g) + 6H2O (g) ΔH = –950kJ
The reaction is reversible and exists in dynamic equilibrium where the products reform back the reactants. The following factors are used to increase the yield/amount of Nitrogen (II) oxide:
(i)Removing Nitrogen (II) oxide gas once formed shift the equilibrium forward to the right to replace the Nitrogen (II) oxide.
More/higher yield of Nitrogen (II) oxide is attained as reactants try to return the equilibrium balance.
(ii)Increase in pressure shift the equilibrium backward to the left where there are less volume/molecules.
Less/lower yield of Nitrogen (II) oxide is attained.
Very low pressures increases the distance between reacting NH3 and O2 molecules.
An optimum pressure of about 9 atmospheres is normally used.
Cooling the mixture condenses the water vapour to liquid water
(iii)Increase in temperature shift the equilibrium backward to the left because the reaction is exothermic (ΔH = -950kJ).
Nitrogen (II) oxide and water vapour formed decomposes back to Ammonia and Oxygen to remove excess heat therefore a less yield of Nitrogen (II) oxide is attained.
Very low temperature decreases the collision frequency of Ammonia and Oxygen and thus the rate of reaction too slow and uneconomical.
An optimum temperature of about 900oC is normally used.
(iv)Platinum can be used as catalyst.
Platinum is very expensive. It is:
-promoted with Rhodium to increase the surface area/area of contact.
-added/coated on the surface of asbestos to form Platonized –asbestos to reduce the amount/quantity used.
The catalyst does not increase the yield of Nitrogen (II) Oxide but it speed up its rate of formation.
Nitrogen (II) oxide formed is passed through an oxidation reaction chamber where more air oxidizes the Nitrogen (II) Oxide to Nitrogen (IV) Oxide gas.
Chemical equation
2NO (g) + O2 (g) -> 2NO2 (g)
Nitrogen (IV) Oxide gas is passed up to meet a downward flow of water in the absorption chamber. The gas reacts with water to form a mixture of Nitric (V) and Nitric (III) acids
Chemical equation.
2NO2 (g) + H2O (l) -> HNO2 (as) + HNO3 (as)
Excess air is bubbled through the mixture to oxidize Nitric (III)/ HNO2 (as) to Nitric (V)/HNO3 (as)
Chemical equation.
O2 (g) + 2HNO2 (as) -> 2HNO3 (as)
Overall chemical equation in the absorption chamber.
O2 (g) + 4NO2 (g) + 2H2O (l) -> 4HNO3 (as)
The acid is 65% concentrated. It is made 100% concentrated by either:
(i) fractional distillation or
(ii) added to concentrated sulphuric (VI) acid to remove the 35% of water.
Oswald’s Process: Manufacture of Nitric (v) Acid