Notes on WATER AND HYDROGEN
Pure water is a colourless, odorless, tasteless, neutral liquid. Pure water does not exist in nature but naturally in varying degree of purity. The main sources of water include rain, springs, borehole, lakes, seas and oceans:
Water is generally used for the following purposes:
(i) Drinking by animals and plants.
(ii) Washing clothes.
(iii) Bleaching and dyeing.
(iv) Generating hydroelectric power.
(v) Cooling industrial processes.
Water dissolves many substances/solutes.
It is therefore called universal solvent.
It contains about 35% dissolved Oxygen which support aquatic fauna and flora.
Water naturally exists in three phases/states solid ice, liquid water and gaseous water vapour.
The three states of water are naturally interconvertible.
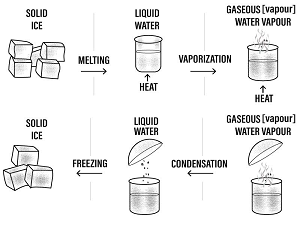
The natural interconvertion of the three phases/states of water forms the water cycle.
Precipitation
Liquid water in land, lakes, seas and oceans use the solar/sun energy to evaporate/vapourize to form water vapour/gas. Solar/sun energy is also used during transpiration by plants and respiration by animals.
During evaporation, the water vapour rises up the earth’s surface. Temperatures decrease with height above the earth surface increase. Water vapour therefore cools as it rises up. At a height where it is cold enough to below 373Kelvin/100oC Water vapour looses enough energy to form tiny droplets of liquid.
The process by which a gas/water vapour changes to a liquid is called condensation/liquidification.
On further cooling, the liquid looses more energy to form ice/solid. The process by which a liquid/water changes to a ice/solid is called freezing/solidification. Minute/tiny ice/solid particles float in the atmosphere and coalesce/join together to form clouds. When the clouds become too heavy they fall to the earth’s surface as rain/snow as the temperature increase with the fall.
Interconversion of the three phases/states water
Solidification
Pure water has:
- fixed/constant/sharp freezing point/melting point of 273K/0oC
- fixed/constant/sharp boiling point of 373K/100oC at sea level/1 atmosphere pressure
- fixed density of 1gcm-3
This is the criteria of identifying pure/purity of water.
Whether a substance is water can be determined by using the following methods:
Experiment to test for presence of water using anhydrous copper (II) suphate (VI)
Procedure
Put about 2g of anhydrous copper (II) sulphate (VI) crystals into a clean test tube. Add three drops of tap water. Repeat the procedure using distilled water.
Observation
Colour changes from white to blue
Explanation
Anhydrous copper (II) sulphate (VI) is white. On adding water, anhydrous copper (II) sulphate (VI) gains/reacts with water to form hydrated copper (II) sulphate (VI).
Hydrated copper (II) sulphate (VI) is blue.Hydrated copper (II) sulphate (VI) contains water of crystallization.
The change of white anhydrous copper (II) sulphate (VI) to bluehydrated copper (II) sulphate (VI) is a confirmatory test for the presence of water
Chemical equation
Anhydrous Hydrated copper (II) sulphate (VI) + Water -> copper (II) sulphate (VI)
(white) (blue)
CuSO4(s) + 5H2O (l) -> CuSO4.5H2O(s)
b) To test for presence of water using anhydrous cobalt (II) chloride
Procedure
Put about 5cm3 of water into a clean test tube.
Dip a dry anhydrouscobalt (II) chloride paper into the test tube.
Repeat the procedure using distilled water.
Observation
Colour changes from blue to pink
Explanation
Anhydrous cobalt (II) chloride is blue. On adding water, anhydrous cobalt (II) chloride gains/reacts with water to form hydrated cobalt (II) chloride.
Hydrated cobalt (II) chloride is pink.
Hydrated cobalt (II) chloride contains water of crystallization.
The change of blue anhydrous cobalt (II) chloride to pinkhydrated cobalt (II) chloride is a confirmatory test for the presence of water Chemical equation.
Anhydrous Hydrated cobalt (II) chloride + Water -> cobalt (II) chloride
(Blue) (pink)
CoCl2 (s) + 5H2O (l) -> CoCl2.5H2O(s)
Burning a candle in air
Most organic substances/fuels burn in air to produce water. Carbon (IV) oxide gas is also produced if the air is sufficient/excess.
Procedure
Put about 2g of anhydrous copper (II) sulphate (VI) crystals in a boiling tube.
Put about 5cm3 of lime water in a boiling tube.
Light a small candle stick. Place it below an inverted thistle/filter funnel
Collect the products of the burning candle by setting the apparatus as below
Set up of apparatus
Observation
The sanction pump pulls the products of burning into the inverted funnel. Colour of anhydrous copper (II) sulphate (VI) changes from white to blue. A white precipitate is formed in the lime water/calcium hydroxide.
Explanation
When a candle burn it forms a water and carbon (IV) oxide.
Water turns anhydrous copper (II) sulphate (VI) changes from white to blue.
Carbon (IV) oxide gasforms white precipitate when bubbled in lime water/calcium hydroxide.
Since:
(i) hydrogen in the wax burn to form water
Hydrogen + Oxygen -> Water
(from candle) (from the air)
2H2 (g) + O2 (g) -> 2H2O (g/l)
(ii) carbon in the wax burn to form carbon (IV) oxide
Hydrogen + Oxygen -> Water
(from candle) (from the air)
C(s) + O2(g) -> CO2 (g)
The candle before burning therefore contained only Carbon and Hydrogen only. A compound made up of hydrogen and carbon is called Hydrocarbon.
A candle is a hydrocarbon.
Other hydrocarbons include: Petrol, diesel, Kerosene, and Laboratory gas. Hydrocarbons burn in air to form water and carbon (IV) oxide gas.
Hydrocarbons + Oxygen -> Water + Oxygen
Water pollution
Water pollution takes place when undesirable substances are added into the water. Sources of water pollution include:
(i)Industrial chemicals being disposed into water bodies like rivers, lakes and oceans.
(ii)Discharging untreated /raw sewage into water bodies.
(iii)Leaching of insecticides/herbicides form agricultural activities into water bodies.
(iv)Discharging non-biodegradable detergents after domestic and industrial use into water bodies.
(v)Petroleum oil spilling by ships and oil refineries
(vi)Toxic/poisonous gases from industries dissolving in rain.
(vii) Acidic gases from industries dissolving in rain to form “acid rain”
(viii)Discharging hot water into water bodies. This reduces the quantity of dissolved Oxygen in the water killing the aquatic fauna and flora.
Water pollution can be reduced by:
(i) Reducing the use of agricultural fertilizers and chemicals in agricultural activities.
(ii) Use of biological control method instead of insecticides and herbicides
(iii) Using biodegradable detergents
REACTION OF WATER WITH METALS.
Some metals react with water while others do not. The reaction of metals with water depends on the reactivity series. The higher the metal in the reactivity series the more reactive the metal with water .The following experiments shows the reaction of metals with cold water and water vapour/steam.
(a)Reaction of sodium/ potassium with cold water:
Procedure
Put about 500cm3 of water in a beaker. Add three drops of phenolphthalein indicator/litmus solution/universal indicator solution/methyl orange indicator into the water.
Cut a very small piece of sodium .Using a pair of forceps put the metal into the water.
Observation
Sodium melts to a silvery ball that floats and darts on the surface decreasing in size. Effervescence/fizzing/ bubbles of colourless gas produced.
Colour of phenolphthalein turns pink
Colour of litmus solution turns blue
Colour of methyl orange solution turns Orange
Colour of universal indicator solution turns blue
Explanation
Sodium is less dense than water. Sodium floats on water and vigorously reacts to form an alkaline solution of sodium hydroxide and producing hydrogen gas. Sodium is thus stored in paraffin to prevent contact with water.
Chemical equation
Sodium + Water -> Sodium hydroxide + Hydrogen gas
2Na(s) + 2H2O (l) -> 2NaOH (aq) + H2(g)
To collect hydrogen gas, Sodium metal is forced to sink to the bottom of the trough/beaker by wrapping it in wire gauze/mesh.
Potassium is more reactive than Sodium. On contact with water it explodes/burst into flames. An alkaline solution of potassium hydroxide is formed and hydrogen gas
Chemical equation
Potassium + Water -> Potassium hydroxide + Hydrogen gas
2K(s) + 2H2O (l) -> 2KOH (aq) + H2(g)
Caution: Reaction of Potassium with water is very risky to try in a school laboratory.
(b)Reaction of Lithium/ Calcium with cold water:
Procedure
Put about 200cm3 of water in a beaker. Add three drops of phenolphthalein indicator/litmus solution/universal indicator solution/methyl orange indicator into the water.
Cut a small piece of Lithium .Using a pair of forceps put the metal into the water.
Repeat with a piece Calcium metal
Observation
Lithium sinks to the bottom of the water. Rapid effervescence/fizzing/ bubbles of colourless gas produced.
Colour of phenolphthalein turns pink
Colour of litmus solution turns blue
Colour of methyl orange solution turns Orange
Colour of universal indicator solution turns blue
Explanation
Lithium and calcium are denser than water. Both sink in water and vigorously react to form an alkaline solution of Lithium hydroxide / calcium hydroxide and producing hydrogen gas. Lithium is more reactive than calcium. It is also stored in paraffin like Sodium to prevent contact with water.
Chemical equation
Lithium + Water -> Lithium hydroxide + Hydrogen gas
2Li(s) + 2H2O (l) -> 2LiOH (aq) + H2 (g)
Calcium + Water -> Calcium hydroxide + Hydrogen gas
Ca(s) + 2H2O (l) -> Ca (OH) 2(aq) + H2 (g)
(c) Reaction of Magnesium/Zinc/ Iron with Steam/water vapour:
Procedure method1
Place some wet sand or cotton/glass wool soaked in water at the bottom of an ignition/hard glass boiling tube.
Polish magnesium ribbon using sand paper.
Coil it at the centre of the ignition/hard glass boiling tube.
Set up the apparatus as below.
Heat the wet sand or cotton/glass wool soaked in water gently to:
(i) Drive away air in the ignition/hard glass boiling tube.
(ii) Generate steam
Heat the coiled ribbon strongly using another burner. Repeat the experiment using Zinc powder and fresh Iron filings.
Set up of apparatus
Observations
(i)With Magnesium ribbon:
The Magnesium glows with a bright flame (and continues to burn even if heating is stopped)
White solid /ash formed
White solid /ash formed dissolve in water to form a colourless solution
Colourless gas produced/collected that extinguish burning splint with “pop sound”
(ii) With Zinc powder:
The Zinc powder turns red hot on strong heating
Yellow solid formed that turn white on cooling
White solid formed on cooling does not dissolve in water.
(iii)With Iron fillings:
The Iron fillings turn red hot on strong heating
Dark blue solid formed
Dark blue solid formed does not dissolve in water.
Procedure method 2
Put some water in a round bottomed flask
Polish magnesium ribbon using sand paper.
Coil it at the centre of a hard glass tube
Set up the apparatus as below.
Heat water strongly to boil so as to:
(i) drive away air in the glass tube.
(ii) generate steam
Heat the coiled ribbon strongly using another burner. Repeat the experiment using Zinc powder and fresh Iron filings.
Observations
(i)With Magnesium ribbon:
The Magnesium glows with a bright flame (and continues to burn even if heating is stopped)
White solid /ash formed
White solid /ash formed dissolve in water to form a colourless solution
Colourless gas produced/collected that extinguish burning splint with “pop sound”
(ii) With Zinc powder:
The Zinc powder turns red hot on strong heating
Yellow solid formed that turn white on cooling
White solid formed on cooling does not dissolve in water.
(iii)With Iron fillings:
The Iron fillings turn red hot on strong heating
Dark blue solid formed
Dark blue solid formed does not dissolve in water.
Explanations
(a)Hot magnesium burn vigorously in steam. The reaction is highly exothermic generating enough heat/energy to proceed without further heating.
White Magnesium oxide solid/ash is left as residue.
Hydrogen gas is produced .It extinguishes a burning splint with a “pop sound”.
Chemical Equation
Magnesium + Steam -> Magnesium oxide + Hydrogen
Mg(s) + H2O(g) -> MgO(s) + H2(g)
Magnesium oxide reacts /dissolves in water to form an alkaline solution of Magnesium oxide
Chemical Equation
Magnesium oxide + Water -> Magnesium hydroxide
MgO(s) + H2O(l) -> Mg(OH) 2 (aq)
(b)Hot Zinc react vigorously in steam forming yellow Zinc oxide solid/ash as residue which cools to white.
Hydrogen gas is produced .It extinguishes a burning splint with a “pop sound”.
Chemical Equation
Zinc + Steam -> Zinc oxide + Hydrogen
Zn(s) + H2O(g) -> ZnO(s) + H2(g)
Zinc oxide does not dissolve in water.
(c)Hot Iron reacts with steam forming dark blue tri iron tetra oxide solid/ash as residue.
Hydrogen gas is produced .It extinguishes a burning splint with a “pop sound”.
Chemical Equation
Iron + Steam -> Tri iron tetra oxide + Hydrogen
2Fe(s) + 4H2O(g -> Fe2O4(s) + 4H2(g)
Tri iron tetra oxide does not dissolve in water.
(d)Aluminum reacts with steam forming an insoluble coat/cover of impervious layer of aluminum oxide on the surface preventing further reaction. (e) Lead, Copper, Mercury, Silver, Gold and Platinum do not react with either water or steam.
