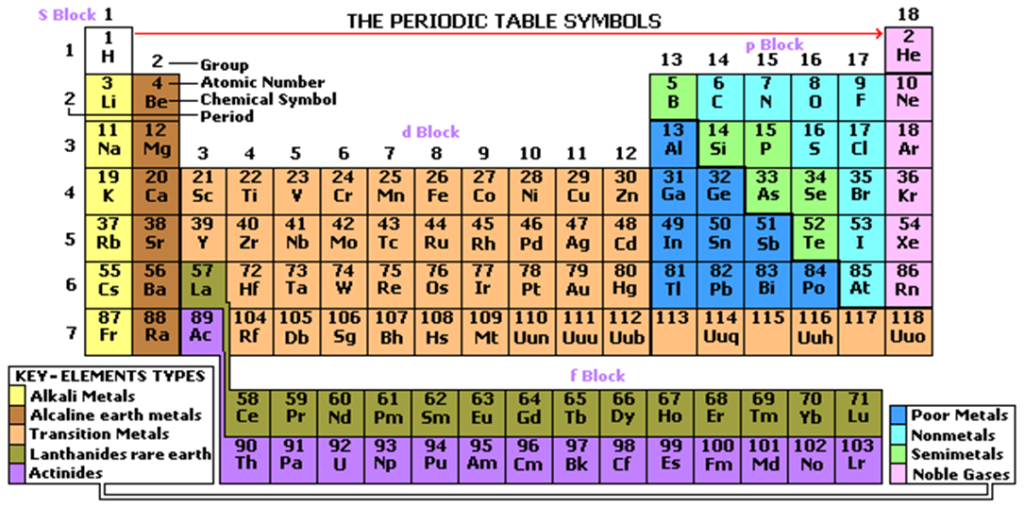
There are over 100 elements so far discovered. Scientists have tried to group them together in a periodic table.
A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers.
This table was successfully arranged in 1913 by the British scientist Henry Moseley from the previous work of the Russian Scientist Dmitri Mendeleev.
The horizontal arrangement forms period. Atoms in the same period have the same the same number of energy levels in their electronic structure. i.e.
The number of energy levels in the electronic configuration of an element determine the period to which the element is in the periodic table.
e.g.
Which period of the periodic table are the following isotopes/elements/atoms?
- 126C
Electron structure 2:4 => 2 energy levels used thus Period 2
- 2311Na
Electron structure 2:8:1 => 3 energy levels used thus Period 3
- 3919K
Electron structure 2:8:8:1 => 4 energy levels used thus Period 4
- 11H
Electron structure 1: => 1 energy level used thus Period 1
The vertical arrangement of elements forms a group. Atoms in the same have the same the same group have the same number of outer energy level electrons as per their electronic structure. i.e.
The number of electrons in the outer energy level an element determine the group to which the element is, in the periodic table.
- 126C
Electron structure 2:4 => 4 electrons in outer energy level thus Group IV
- 2311C
Electron structure 2:8:1 => 1 electron in outer energy level thus Group I
- 3919K
Electron structure 2:8:8:1=>1 electron in outer energy level thus Group I
- 11H
Electron structure 1: => 1 electron in outer energy level thus Group I
By convention;
(i)Periods are named using English numerals 1, 2, 3, 4…
(ii)Groups are named using Roman numerals I, II, III, IV…
There are eighteen groups in a standard periodic table. There are seven periods in a standard periodic table
When an atom has maximum number of electrons in its outer energy level, it is said to be stable.
When an atom has no maximum number of electrons in its outer energy level, it is said to be unstable.
All stable atoms are in group 8/18 of the periodic table.All other elements are unstable.
All unstable atoms/isotopes try to be stable through chemical reactions. A chemical reaction involves gaining or losing outer electrons (electron transfer) .When electron transfer take place, an ion is formed.
An ion is formed when an unstable atom gain or donate electrons in its outer energy level in order to be stable. Whether an atom gain or donate electrons depend on the relative energy required to donate or gain extra electrons i.e.
Examples
- 199 F has electronic structure/configuration 2:7.
It can donate the seven outer electrons to have stable electronic structure/configuration 2:.
It can gain one extra electron to have stable electronic structure/configuration 2:8. Gaining requires less energy, and thus Fluorine reacts by gaining one extra electrons.
- 2313 Al has electronic structure/configuration 2:8:3
It can donate the three outer electrons to have stable electronic structure/configuration 2:8.
It can gain five extra electrons to have stable electronic structure/configuration 2:8:8. Donating requires less energy, and thus Aluminium reacts by donating its three outer electrons.
Elements with less than four electrons in the outer energy level donates /lose the outer electrons to be stable and form a positively charged ion called cation.
A cation therefore has more protons (positive charge) than electrons (negative charge)
Generally metals usually form cation
Elements with more than four electrons in the outer energy level gain /acquire extra electrons in the outer energy level to be stable and form a negatively charged ion called anion.
An anion therefore has fewer protons (positive charge) than electrons (negative charge)
Generally non metals usually form anion. Except Hydrogen
The charge carried by an ion is equal to the number of electrons gained/acquired or donated/lost.
Examples of ion formation
1.11H
H -> H+ + e
(atom) (monovalent cation) (electrons donated/lost)
Electronic configuration 1: (No electrons remains)
2. 2713 Al
Al -> Al3+ + 3e
(atom) (trivalent cation) (3 electrons donated/lost)
Electron 2:8:3 2:8
structure (unstable) (stable)
3. 2311 Na
Na -> Na+ + e
(atom) (cation) ( 1 electrons donated/lost)
Electron 2:8:1 2:8
structure (unstable) (stable)
4. 2412Mg
Mg -> Mg2+ + 2e
(atom) (cation) ( 2 electrons donated/lost)
Electron 2:8:1 2:8
structure (unstable) (stable)
5. 168O
O + 2e -> O2-
(atom) ( 2 electrons gained/acquired) (anion)
Electron 2:6 2:8
structure (unstable) (stable)
6. 147N
N + 3e -> N3-
(atom) ( 3 electrons gained/acquired) (anion)
Electron 2:5 2:8
structure (unstable) (stable)
7. 3115P
P + 3e -> P3-
(atom) ( 3 electrons gained/acquired) (anion)
Electron 2:5 2:8
structure (unstable) (stable)
8. 199F
F + e -> F–
(atom) ( 1 electrons gained/acquired) (anion)
Electron 2:7 2:8
structure (unstable) (stable)
9. 3517Cl
Cl + e -> Cl–
(atom) ( 1 electrons gained/acquired) (anion)
Electron 2:8:7 2:8:8
structure (unstable) (stable)
3. 3919 K
K -> K+ + e
(atom) (cation) ( 1 electrons donated/lost)
Electron 2:8:8:1 2:8:8
structure (unstable) (stable)
When an element donates/loses its outer electrons, the process is called oxidation. When an element acquires/gains extra electrons in its outer energy level, the process is called reduction. The charge carried by an atom, cation or anion is its oxidation state.
Table showing the oxidation states of some isotopes
| Element | Symbol of element / isotopes | Charge of ion | Oxidation state |
| Hydrogen | 11H 21H(deuterium) 31H(Tritium) | H+ H+ H+ | +1 +1 +1 |
| Chlorine | 3517Cl 3717Cl | Cl– Cl– | -1 -1 |
| Potassium | 3919K 4019K 4119K | K+ K+ K+ | +1 +1 +1 |
| Oxygen | 168O 188O | O2- O2- | -2 -2 |
| Magnesium | 2412Mg | Mg2+ | +2 |
| sodium | 2311Na | Na+ | +1 |
| Copper | Cu | Cu+ Cu2+ | +1 +2 |
| Iron | Fe2+ Fe3+ | +2 +3 | |
| Lead | Pb2+ Pb4+ | +2 +4 | |
| Manganese | Mn2+ Mn7+ | +2 +7 | |
| Chromium | Cr3+ Cr6+ | +3 +6 | |
| Sulphur | S4+ S6+ | +4 +6 | |
| Carbon | C2+ C4+ | +2 +4 |
Note:
Some elements can exist in more than one oxidation state. They are said to have variable oxidation state.
Roman capital numeral is used to indicate the oxidation state of an element with a variable oxidation state in a compound.
Examples:
- Copper (I) means Cu+ as in Copper(I)oxide
- Copper (II) means Cu2+ as in Copper(II)oxide
- Iron (II) means Fe2+ as in Iron(II)sulphide
(iv) Iron (III) means Fe3+ as in Iron(III)chloride
- Sulphur(VI)mean S6+ as in Iron(III)sulphate(VI)
- Sulphur(VI)mean S6+ as in sulphur(VI)oxide
- Sulphur(IV)mean S4+ as in sulphur(IV)oxide
- Sulphur(IV)mean S4+ as in sodium sulphate(IV)
(ix) Carbon(IV)mean C4+ as in carbon(IV)oxide
(x) Carbon(IV)mean C4+ as in Lead(II)carbonate(IV)
(xi) Carbon(II)mean C2+ as in carbon(II)oxide
(xii) Manganese(IV)mean Mn4+ as in Manganese(IV)oxide
A compound is a combination of two or more elements in fixed proportions. The ratio of the atoms making a compound is called the chemical formulae. Elements combine together to form a compound depending on their combining power.
The combining power of atoms in an element is called Valency. Valency of an element is equal to the number of:
(i) Hydrogen atoms that an atom of element can combine with or displace.
(ii) Electrons gained /acquired in outer energy level by non metals to be stable/attain duplet/octet.
(iii) Electrons donated/lost by outer energy level of metals to be stable/attain octet/duplet.
(iv) Charges carried by ions/cations/ions
Group of atoms that react as a unit during chemical reactions are called radicals. Elements with variable oxidation state also have more than one valency.
Table showing the valency of common radicals
| Radical name | Chemical formulae | Combining power / Valency |
| Ammonium | NH4 + | 1 |
| Hydroxide | OH– | 1 |
| Nitrate(V) | NO3 – | 1 |
| Hydrogen carbonate | HCO3– | 1 |
| Hydrogen sulphate(VI) | HSO4– | 1 |
| Hydrogen sulphate(IV) | HSO3– | 1 |
| Manganate(VII) | MnO4– | 1 |
| Chromate(VI) | CrO42- | 2 |
| Dichromate(VI) | Cr2O72- | 2 |
| Sulphate(VI) | SO42- | 2 |
| Sulphate(IV) | SO32- | 2 |
| Carbonate(IV) | CO32- | 2 |
| Phosphate(V) | PO42- | 3 |
Table showing the valency of some common metal and non metals
| Element/metal | Valency | Element/non metal | Valency |
| Hydrogen | 1 | Florine | 1 |
| Lithium | 1 | Chlorine | 1 |
| Beryllium | 2 | Bromine | 1 |
| Boron | 3 | Iodine | 1 |
| Sodium | 1 | Carbon | 4 |
| Magnesium | 2 | Nitrogen | 3 |
| Aluminium | 3 | Oxygen | 2 |
| Potassium | 1 | Phosphorus | 3 |
| Calcium | 2 | ||
| Zinc | 2 | ||
| Barium | 2 | ||
| Mercury | 2 | ||
| Iron | 2 and 3 | ||
| Copper | 1 and 2 | ||
| Manganese | 2 and 4 | ||
| Lead | 2 and 4 |
From the valency of elements, the chemical formular of a compound can be derived using the following procedure:
(i)Identify the elements and radicals making the compound
(ii)Write the symbol/formular of the elements making the compound starting with the metallic element
(iii)Assign the valency of each element /radical as superscript.
(iv)Interchange/exchange the valencies of each element as subscript.
(v)Divide by the smallest/lowest valency to derive the smallest whole number ratios
Ignore a valency of 1.
This is the chemical formula.
Practice examples
Write the chemical formula of
(a)Aluminium oxide
| Elements making compound | Aluminium | Oxygen |
| Symbol of elements/radicals in compound | Al | O |
| Assign valencies as superscript | Al3 | O2 |
| Exchange/Interchange the valencies as subscript | Al2 | O3 |
| Divide by smallest valency to get whole number | – | – |
Chemical formula of Aluminium oxide is thus: Al2 O3
This means: 2atoms of Aluminium combine with 3 atoms of Oxygen
(b)Sodium oxide
| Elements making compound | Sodium | Oxygen |
| Symbol of elements/radicals in compound | Na | O |
| Assign valencies as superscript | Na1 | O2 |
| Exchange/Interchange the valencies as subscript | Na2 | O1 |
| Divide by smallest valency to get whole number | – | – |
Chemical formula of Sodium oxide is thus: Na2 O
This means: 2atoms of Sodium combine with 1 atom of Oxygen
(c)Calcium oxide
| Elements making compound | Calcium | Oxygen |
| Symbol of elements/radicals in compound | Ca | O |
| Assign valencies as superscript | Ca2 | O2 |
| Exchange/Interchange the valencies as subscript | Ca2 | O2 |
| Divide by two to get smallest whole number ratio | Ca1 | O1 |
Chemical formula of Calcium oxide is thus: CaO
This means: 1 atom of calcium combine with 1 atom of Oxygen.
(d)Lead (IV) oxide
| Elements making compound | Lead | Oxygen |
| Symbol of elements/radicals in compound | Pb | O |
| Assign valencies as superscript | Pb4 | O2 |
| Exchange/Interchange the valencies as subscript | Pb2 | O4 |
| Divide by two to get smallest whole number ratio | Pb1 | O2 |
Chemical formula of Lead (IV) oxide is thus: PbO2
This means: 1 atom of lead combine with 2 atoms of Oxygen.
(e)Lead (II) oxide
| Elements making compound | Lead | Oxygen |
| Symbol of elements/radicals in compound | Pb | O |
| Assign valencies as superscript | Pb2 | O2 |
| Exchange/Interchange the valencies as subscript | Pb2 | O2 |
| Divide by two to get smallest whole number ratio | Pb1 | O1 |
Chemical formula of Lead (II) oxide is thus: PbO
This means: 1 atom of lead combine with 1 atom of Oxygen.
(e)Iron (III) oxide
| Elements making compound | Iron | Oxygen |
| Symbol of elements/radicals in compound | Fe | O |
| Assign valencies as superscript | Fe3 | O2 |
| Exchange/Interchange the valencies as subscript | Fe2 | O3 |
| Divide by two to get smallest whole number ratio | – | – |
Chemical formula of Iron(III) oxide is thus: Fe2O3
This means: 2 atom of lead combine with 3 atom of Oxygen.
(f)Iron (II) sulphate (VI)
| Elements making compound | Iron | sulphate(VI) |
| Symbol of elements/radicals in compound | Fe | SO4 |
| Assign valencies as superscript | Fe2 | SO4 2 |
| Exchange/Interchange the valencies as subscript | Fe2 | SO4 2 |
| Divide by two to get smallest whole number ratio | Fe1 | SO4 1 |
Chemical formula of Iron (II) sulphate (VI) is thus: FeSO4
This means: 1 atom of Iron combine with 1 sulphate (VI) radical.
(g)Copper (II) sulphate (VI)
| Elements making compound | Copper | sulphate(VI) |
| Symbol of elements/radicals in compound | Cu | SO4 |
| Assign valencies as superscript | Cu2 | SO4 2 |
| Exchange/Interchange the valencies as subscript | Cu2 | SO4 2 |
| Divide by two to get smallest whole number ratio | Cu1 | SO4 1 |
Chemical formula of Cu(II)sulphate(VI) is thus: CuSO4
This means: 1 atom of Copper combine with 1 sulphate (VI) radical.
(h)Aluminium sulphate (VI)
| Elements making compound | Aluminium | sulphate(VI) |
| Symbol of elements/radicals in compound | Al | SO4 |
| Assign valencies as superscript | Al3 | SO4 2 |
| Exchange/Interchange the valencies as subscript | Al2 | SO4 3 |
| Divide by two to get smallest whole number ratio | – | – |
Chemical formula of Aluminium sulphate (VI) is thus: Al2(SO4)3
This means: 2 atom of Aluminium combine with 3 sulphate (VI) radical.
(i)Aluminium nitrate (V)
| Elements making compound | Aluminium | nitrate(V) |
| Symbol of elements/radicals in compound | Al | NO3 |
| Assign valencies as superscript | Al3 | NO3 1 |
| Exchange/Interchange the valencies as subscript | Al1 | NO3 3 |
| Divide by two to get smallest whole number ratio | – | – |
Chemical formula of Aluminium sulphate (VI) is thus: Al (NO3)3
This means: 1 atom of Aluminium combine with 3 nitrate (V) radical.
(j)Potassium manganate (VII)
| Elements making compound | Potassium | manganate(VII) | |
| Symbol of elements/radicals in compound | K | MnO4 | |
| Assign valencies as superscript | K 1 | MnO4 1 | |
| Exchange/Interchange the valencies as subscript | K1 | MnO4 1 | |
| Divide by two to get smallest whole number ratio | – | – | |
Chemical formula of Potassium manganate (VII) is thus: KMnO4
This means: 1 atom of Potassium combine with 4 manganate (VII) radical.
(k)Sodium dichromate (VI)
| Elements making compound | Sodium | dichromate(VI) | |
| Symbol of elements/radicals in compound | Na | Cr2O7 | |
| Assign valencies as superscript | Na 1 | Cr2O7 2 | |
| Exchange/Interchange the valencies as subscript | Na2 | Cr2O7 1 | |
| Divide by two to get smallest whole number ratio | – | – | |
Chemical formula of Sodium dichromate (VI) is thus: Na2 Cr2O7
This means: 2 atom of Sodium combine with 1 dichromate (VI) radical.
(l)Calcium hydrogen carbonate
| Elements making compound | Calcium | Hydrogen carbonate | |
| Symbol of elements/radicals in compound | Ca | CO3 | |
| Assign valencies as superscript | Ca 2 | HCO3 1 | |
| Exchange/Interchange the valencies as subscript | Ca1 | HCO3 2 | |
| Divide by two to get smallest whole number ratio | – | – | |
Chemical formula of Calcium hydrogen carbonate is thus: Ca (HCO3)2
This means: 1 atom of Calcium combine with 2 hydrogen carbonate radical.
(l)Magnesium hydrogen sulphate (VI)
| Elements making compound | Magnesium | Hydrogen sulphate(VI) | |
| Symbol of elements/radicals in compound | Mg | HSO4 | |
| Assign valencies as superscript | Mg 2 | HSO4 1 | |
| Exchange/Interchange the valencies as subscript | Mg1 | HSO4 2 | |
| Divide by two to get smallest whole number ratio | – | – | |
Chemical formula of Magnesium hydrogen sulphate (VI) is thus: Mg (HSO4)2
This means: 1 atom of Magnesium combine with 2 hydrogen sulphate (VI) radical.
Compounds are formed from chemical reactions. A chemical reaction is formed when atoms of the reactants break free to bond again and form products. A chemical reaction is a statement showing the movement of reactants to form products. The following procedure is used in writing chemical equations:
1. Write the word equation
2. Write the correct chemical formula for each of the reactants and products
3. Check if the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side.
4. Multiply the chemical formula containing the unbalanced atoms with the lowest common multiple if the number of atoms on one side is not equal. This is called balancing.
Do not change the chemical formula of the products/reactants.
5. Assign in brackets, the physical state/state symbols of the reactants and products after each chemical formula as:
(i) (s) for solids
(ii) (l) for liquids
(iii) (g) for gas
(iv) (aq) for aqueous/dissolved in water to make a solution.
Practice examples
Write a balanced chemical equation for the following
- Hydrogen gas is prepared from reacting Zinc granules with dilute hydrochloric acid.
Procedure
1. Write the word equation
Zinc + Hydrochloric acid -> Zinc chloride + hydrogen gas
2. Write the correct chemical formula for each of the reactants and products
Zn + HCl -> ZnCl2 + H2
3. Check if the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side.
Number of atoms of Zn on the reactant side is equal to product side
One atom of H in HCl on the reactant side is not equal to two atoms in H2 on product side.
One atom of Cl in HCl on the reactant side is not equal to two atoms in ZnCl2 on product side.
4. Multiply the chemical formula containing the unbalanced atoms with the lowest common multiple if the number of atoms on one side is not equal.
Multiply HCl by “2” to get “2” Hydrogen and “2” Chlorine on product and reactant side.
Zn + 2 HCl -> ZnCl2 + H2
5. Assign in brackets, the physical state/state symbols .
Zn(s) + 2 HCl(aq) -> ZnCl2 (aq) + H2(g)
- Oxygen gas is prepared from decomposition of Hydrogen peroxide solution to water
Procedure
1. Write the word equation
Hydrogen peroxide -> Water + oxygen gas
2. Write the correct chemical formula for each of the reactants and products
H2O2 -> H2O + O2
3. Check if the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side.
Number of atoms of H on the reactant side is equal to product side
Two atom of O in H2O2 on the reactant side is not equal to three atoms (one in H2O and two in O2) on product side.
4. Multiply the chemical formula containing the unbalanced atoms with the lowest common multiple if the number of atoms on one side is not equal.
Multiply H2O2 by “2” to get “4” Hydrogen and “4” Oxygen on reactants
Multiply H2O by “2” to get “4” Hydrogen and “2” Oxygen on product side
When the “2” Oxygen in O2 and the“2” in H2O are added on product side they are equal to the“4” Oxygen on reactants side.
2H2O2 -> 2H2O + O2
5. Assign in brackets, the physical state/state symbols.
2H2O2(aq) -> 2H2O(l) + O2(g)
- Chlorine gas is prepared from Potassium manganate (VII) reacting with hydrochloric acid to form potassium chloride solution, manganese (II) chloride solution, water and chlorine gas.
Procedure
1. Write the word equation
Potassium manganate (VII) + Hydrochloric acid ->
Potassium chloride + manganese (II) chloride + chlorine +water
2. Write the correct chemical formula for each of the reactants and products
KMnO4 + HCl -> KCl + MnCl2 +H2O + Cl2
3. Check if the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side.
Number of atoms of K and Mn on the reactant side is equal to product side
Two atom of H in H2O on the product side is not equal to one atom on reactant side.
Four atom of O in KMnO4 is not equal to one in H2O
One atom of Cl in HCl on reactant side is not equal to three (one in H2O and two in Cl2)
4. Multiply the chemical formula containing the unbalanced atoms with the lowest common multiple if the number of atoms on one side is not equal.
Multiply HCl by “16” to get “16” Hydrogen and “16” Chlorine on reactants
Multiply KMnO4by “2” to get “2” Potassium and “2” manganese, “2 x4 =8” Oxygen on reactant side.
Balance the product side to get:
2 KMnO4 +16 HCl -> 2 KCl + 2 MnCl2 +8 H2O + 5 Cl2
5. Assign in brackets, the physical state/state symbols.
2KMnO4(s) +16 HCl(aq)-> 2 KCl (aq) + 2MnCl2(aq)+8 H2O(l)+5 Cl2(g)
(d)Carbon (IV) oxide gas is prepared from Calcium carbonate reacting with hydrochloric acid to form calcium chloride solution, water and carbon (IV) oxide gas.
Procedure
1. Write the word equation
Calcium carbonate + Hydrochloric acid ->
Calcium chloride solution+ water +carbon (IV)oxide
2. Write the correct chemical formula for each of the reactants and products
CaCO3 + HCl -> CaCl2 +H2O + CO2
3. Check if the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side.
4. Multiply the chemical formula containing the unbalanced atoms with the lowest common multiple if the number of atoms on one side is not equal.
5. Assign in brackets, the physical state/state symbols.
CaCO3(s) + 2 HCl(aq) -> CaCl2(aq) + H2O(l) + CO2(g)
(d)Sodium hydroxide solution neutralizes hydrochloric acid to form salt and water.
NaOH(aq) + HCl(aq) -> NaCl (aq) + H2O(l)
(e)Sodium reacts with water to form sodium hydroxide and hydrogen gas.
2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2(g)
(f)Calcium reacts with water to form calcium hydroxide and hydrogen gas
Ca(s) + 2H2O(l) -> Ca(OH)2(aq) + H2(g)
(g)Copper (II) Oxide solid reacts with dilute hydrochloric acid to form copper (II) chloride and water.
CuO(s) + 2HCl(aq) -> CuCl2(aq) + H2O(l)
(h)Hydrogen sulphide reacts with Oxygen to form sulphur (IV) Oxide and water.
2H2S(g) + 3O2(g) -> 2SO2(g) + 2H2O(l)
(i)Magnesium reacts with steam to form Magnesium Oxide and Hydrogen gas.
Mg(s) + 2H2O(g) -> MgO(s) + H2(g)
(j)Ethane (C2H6) gas burns in air to form Carbon (IV) Oxide and water.
2C2H6(g) + 7O2(g) -> 4CO2(g) + 6H2O(l)
(k)Ethene (C2H4) gas burns in air to form Carbon (IV) Oxide and water.
C2H4(g) + 3O2(g) -> 2CO2(g) + 2H2O(l)
(l)Ethyne (C2H2) gas burns in air to form Carbon (IV) Oxide and water.
2C2H2(g) + 5O2(g) -> 4CO2(g) + 2H2O(l)
