The number of valence electrons and the number of occupied energy levels in an atom of an element determine the position of an element in the periodic table .i.e
The number of occupied energy levels determines the Period and the valence electrons determine the Group.
Elements in the same group have similar physical and chemical properties. The trends in physical and chemical properties of elements in the same group vary down the group. Elements in the same group thus constitute a chemical family.
- Group I elements: Alkali metals
Group I elements are called Alkali metals except Hydrogen which is a non metal. The alkali metals include:
| Element | Symbol | Atomic number | Electron structure | Oxidation state | Valency |
| Lithium | Li | 3 | 2:1 | Li+ | 1 |
| Sodium | Na | 11 | 2:8:1 | Na+ | 1 |
| Potassium | K | 19 | 2:8:8:1 | K+ | 1 |
| Rubidium | Rb | 37 | 2:8:18:8:1 | Rb+ | 1 |
| Caesium | Cs | 55 | 2:8:18:18:8:1 | Cs+ | 1 |
| Francium | Fr | 87 | 2:8:18:32:18:8:1 | Fr+ | 1 |
All alkali metals atom has one electron in the outer energy level. They therefore are monovalent. They donate /lose the outer electron to have oxidation state M+
The number of energy levels increases down the group from Lithium to Francium. The more the number of energy levels the bigger/larger the atomic size. e.g.
The atomic size of Potassium is bigger/larger than that of sodium because Potassium has more/4 energy levels than sodium (3 energy levels).
Atomic and ionic radius
The distance between the centre of the nucleus of an atom and the outermost energy level occupied by electron/s is called atomic radius. Atomic radius is measured in nanometers(n).The higher /bigger the atomic radius the bigger /larger the atomic size.
The distance between the centre of the nucleus of an ion and the outermost energy level occupied by electron/s is called ionic radius. Ionic radius is also measured in nanometers (n).The higher /bigger the ionic radius the bigger /larger the size of the ion.
Atomic radius and ionic radius depend on the number of energy levels occupied by electrons. The more the number of energy levels the bigger/larger the atomic /ionic radius. e.g.
The atomic radius of Francium is bigger/larger than that of sodium because Francium has more/7 energy levels than sodium (3 energy levels).
Atomic radius and ionic radius of alkali metals increase down the group as the number of energy levels increases.
The atomic radius of alkali metals is bigger than the ionic radius. This is because alkali metals react by losing/donating the outer electron and hence lose the outer energy level.
Table showing the atomic and ionic radius of some alkali metals
| Element | Symbol | Atomic number | Atomic radius(nM) | Ionic radius(nM) |
| Lithium | Li | 3 | 0.133 | 0.060 |
| Sodium | Na | 11 | 0.157 | 0.095 |
| Potassium | K | 19 | 0.203 | 0.133 |
The atomic radius of sodium is 0.157nM .The ionic radius of Na+ is 0.095nM. This is because sodium reacts by donating/losing the outer electrons and hence the outer energy level. The remaining electrons/energy levels experience more effective / greater nuclear attraction/pull towards the nucleus reducing the atomic radius.
Electropositivity
The ease of donating/losing electrons is called electropositivity. All alkali metals are electropositive. Electropositivity increase as atomic radius increase. This is because the effective nuclear attraction on outer electrons decreases with increase in atomic radius. The outer electrons experience less nuclear attraction and can be lost/ donated easily/with ease. Francium is the most electropositive element in the periodic table because it has the highest/biggest atomic radius.
Ionization energy
The minimum amount of energy required to remove an electron from an atom of element in its gaseous state is called 1st ionization energy. The SI unit of ionization energy is kilojoules per mole/kJmole-1 .Ionization energy depend on atomic radius. The higher the atomic radius, the less effective the nuclear attraction on outer electrons/energy level and thus the lower the ionization energy. For alkali metals the 1st ionization energy decrease down the group as the atomic radius increase and the effective nuclear attraction on outer energy level electrons decrease.
e.g. The 1st ionization energy of sodium is 496 kJmole-1 while that of potassium is 419 kJmole-1 .This is because atomic radius increase and thus effective nuclear attraction on outer energy level electrons decrease down the group from sodium to Potassium. It requires therefore less energy to donate/lose outer electrons in Potassium than in sodium.
Physical properties
Soft/Easy to cut: Alkali metals are soft and easy to cut with a knife. The softness and ease of cutting increase down the group from Lithium to Francium. This is because an increase in atomic radius, decreases the strength of metallic bond and the packing of the metallic structure
Appearance: Alkali metals have a shiny grey metallic luster when freshly cut. The surface rapidly/quickly tarnishes on exposure to air. This is because the metal surface rapidly/quickly reacts with elements of air/oxygen.
Melting and boiling points: Alkali metals have a relatively low melting/boiling point than common metals like Iron. This is because alkali metals use only one delocalized electron to form a weak metallic bond/structure.
Electrical/thermal conductivity: Alkali metals are good thermal and electrical conductors. Metals conduct using the outer mobile delocalized electrons. The delocalized electrons move randomly within the metallic structure.
Summary of some physical properties of the 1st three alkali metals
| Alkali metal | Appearance | Ease of cutting | Melting point (oC) | Boiling point (oC) | Conductivity | 1st ionization energy |
| Lithium | Silvery white | Not easy | 180 | 1330 | Good | 520 |
| Sodium | Shiny grey | Easy | 98 | 890 | Good | 496 |
| Potassium | Shiny grey | Very easy | 64 | 774 | Good | 419 |
Chemical properties
(i)Reaction with air/oxygen
On exposure to air, alkali metals react with the elements in the air.
Example
On exposure to air, Sodium first reacts with Oxygen to form sodium oxide.
4Na(s) + O2(g) -> 2Na2O(s)
The sodium oxide formed further reacts with water/moisture in the air to form sodium hydroxide solution.
Na2O(s) + H2O(l) -> 2NaOH(aq)
Sodium hydroxide solution reacts with carbon (IV) oxide in the air to form sodium carbonate.
2NaOH (aq) + CO2 (g) -> Na2CO3 (g) + H2O (l)
(ii)Burning in air/oxygen
Lithium burns in air with a crimson/deep red flame to form Lithium oxide
4Li (s) + O2 (g) -> 2Li2O(s)
Sodium burns in air with a yellow flame to form sodium oxide
4Na (s) + O2 (g) -> 2Na2O(s)
Sodium burns in oxygen with a yellow flame to form sodium peroxide
2Na (s) + O2 (g) -> Na2O2 (s)
Potassium burns in air with a lilac/purple flame to form potassium oxide
4K (s) + O2 (g) -> 2K2O (s)
(iii) Reaction with water:
Experiment
Measure 500 cm3 of water into a beaker.
Put three drops of phenolphthalein indicator.
Put about 0.5g of Lithium metal into the beaker.
Determine the pH of final product
Repeat the experiment using about 0.1 g of Sodium and Potassium.
Caution: Keep a distance
Observations
| Alkali metal | Observations | Comparative speed/rate of the reaction |
| Lithium | -Metal floats in water -rapid effervescence/fizzing/bubbling -colourless gas produced (that extinguishes burning splint with explosion /“pop” sound) -resulting solution turn phenolphthalein indicator pink -pH of solution = 12/13/14 | Moderately vigorous |
| Sodium | -Metal floats in water -very rapid effervescence /fizzing /bubbling -colourless gas produced (that extinguishes burning splint with explosion /“pop” sound) -resulting solution turn phenolphthalein indicator pink -pH of solution = 12/13/14 | Very vigorous |
| Potassium | -Metal floats in water -explosive effervescence /fizzing /bubbling -colourless gas produced (that extinguishes burning splint with explosion /“pop” sound) -resulting solution turn phenolphthalein indicator pink -pH of solution = 12/13/14 | Explosive/burst into flames |
Explanation
Alkali metals are less dense than water. They therefore float in water. They react with water to form a strongly alkaline solution of their hydroxides and producing hydrogen gas. The rate of this reaction increase down the group. i.e. Potassium is more reactive than sodium .Sodium is more reactive than Lithium.
The reactivity increases as electropositivity increases of the alkali increases. This is because as the atomic radius increases, the ease of donating/losing outer electron increases during chemical reactions.
Chemical equations
2Li(s) + 2H2O(l) -> 2LiOH(aq) + H2(g)
2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2(g)
2K(s) + 2H2O(l) -> 2KOH(aq) + H2(g)
2Rb(s) + 2H2O(l) -> 2RbOH(aq) + H2(g)
2Cs(s) + 2H2O(l) -> 2CsOH(aq) + H2(g)
2Fr(s) + 2H2O(l) -> 2FrOH(aq) + H2(g)
Reactivity increase down the group
(iv) Reaction with chlorine:
Experiment
Cut about 0.5g of sodium into a deflagrating spoon with a lid cover. Introduce it on a Bunsen flame until it catches fire. Quickly and carefully lower it into a gas jar containing dry chlorine to cover the gas jar.
Repeat with about 0.5g of Lithium.
Caution: This experiment should be done in fume chamber because chlorine is poisonous /toxic.
Observation
Sodium metal continues to burn with a yellow flame forming white solid/fumes.
Lithium metal continues to burn with a crimson flame forming white solid / fumes.
Alkali metals react with chlorine gas to form the corresponding metal chlorides. The reactivity increase as electropositivity increase down the group from Lithium to Francium. The ease of donating/losing the outer electrons increase as the atomic radius increase and the outer electron is less attracted to the nucleus.
Chemical equations
2Li(s) + Cl2(g) -> 2LiCl(s)
2Na(s) + Cl2(g) -> 2NaCl(s)
2K(s) + Cl2(g) -> 2KCl(s)
2Rb(s) + Cl2(g) -> 2RbCl(s)
2Cs(s) + Cl2(g) -> 2CsCl(s)
2Fr(s) + Cl2(g) -> 2FrCl(s) Reactivity increase down the group
The table below shows some compounds of the 1st three alkali metals
| Lithium | sodium | Potassium | |
| Hydroxide | LiOH | NaOH | KOH |
| Oxide | Li2O | Na2O | K2O |
| Sulphide | Li2S | Na2S | K2S |
| Chloride | LiCl | NaCl | KCl |
| Carbonate | Li2CO3 | Na2CO3 | K2CO3 |
| Nitrate(V) | LiNO3 | NaNO3 | KNO3 |
| Nitrate(III) | – | NaNO2 | KNO2 |
| Sulphate(VI) | Li2SO4 | Na2SO4 | K2SO4 |
| Sulphate(IV) | – | Na2SO3 | K2SO3 |
| Hydrogen carbonate | – | NaHCO3 | KHCO3 |
| Hydrogen sulphate(VI) | – | NaHSO4 | KHSO4 |
| Hydrogen sulphate(IV) | – | NaHSO3 | KHSO3 |
| Phosphate | – | Na3PO4 | K3PO4 |
| Manganate(VI) | – | NaMnO4 | KMnO4 |
| Dichromate(VI) | – | Na2Cr2O7 | K2Cr2O7 |
| Chromate(VI) | – | Na2CrO4 | K2CrO4 |
Some uses of alkali metals include:
(i)Sodium is used in making sodium cyanide for extracting gold from gold ore.
(ii)Sodium chloride is used in seasoning food.
(iii)Molten mixture of sodium and potassium is used as coolant in nuclear reactors.
(iv)Sodium is used in making sodium hydroxide used in making soapy and soapless detergents.
(v)Sodium is used as a reducing agent for the extraction of titanium from Titanium (IV) chloride.
(vi)Lithium is used in making special high strength glasses
(vii)Lithium compounds are used to make dry cells in mobile phones and computer laptops.
Group II elements: Alkaline earth metals
Group II elements are called Alkaline earth metals . The alkaline earth metals include:
| Element | Symbol | Atomic number | Electron structure | Oxidation state | Valency |
| Beryllium | Be | 4 | 2:2 | Be2+ | 2 |
| Magnesium | Mg | 12 | 2:8:2 | Mg2+ | 2 |
| Calcium | Ca | 20 | 2:8:8:2 | Ca2+ | 2 |
| Strontium | Sr | 38 | 2:8:18:8:2 | Sr2+ | 2 |
| Barium | Ba | 56 | 2:8:18:18:8:2 | Ba2+ | 2 |
| Radium | Ra | 88 | 2:8:18:32:18:8:2 | Ra2+ | 2 |
All alkaline earth metal atoms have two electrons in the outer energy level. They therefore are divalent. They donate /lose the two outer electrons to have oxidation state M2+
The number of energy levels increases down the group from Beryllium to Radium. The more the number of energy levels the bigger/larger the atomic size. e.g.
The atomic size/radius of Calcium is bigger/larger than that of Magnesium because Calcium has more/4 energy levels than Magnesium (3 energy levels).
Atomic radius and ionic radius of alkaline earth metals increase down the group as the number of energy levels increases.
The atomic radius of alkaline earth metals is bigger than the ionic radius. This is because they react by losing/donating the two outer electrons and hence lose the outer energy level.
Table showing the atomic and ionic radius of the 1st three alkaline earth metals
| Element | Symbol | Atomic number | Atomic radius(nM) | Ionic radius(nM) |
| Beryllium | Be | 4 | 0.089 | 0.031 |
| Magnesium | Mg | 12 | 0.136 | 0.065 |
| Calcium | Ca | 20 | 0.174 | 0.099 |
The atomic radius of Magnesium is 0.136nM .The ionic radius of Mg2+ is 0.065nM. This is because Magnesium reacts by donating/losing the two outer electrons and hence the outer energy level. The remaining electrons/energy levels experience more effective / greater nuclear attraction/pull towards the nucleus reducing the atomic radius.
Electropositivity
All alkaline earth metals are also electropositive like alkali metals. The electropositivity increase with increase in atomic radius/size. Calcium is more electropositive than Magnesium. This is because the effective nuclear attraction on outer electrons decreases with increase in atomic radius. The two outer electrons in calcium experience less nuclear attraction and can be lost/ donated easily/with ease because of the higher/bigger atomic radius.
Ionization energy
For alkaline earth metals the 1st ionization energy decrease down the group as the atomic radius increase and the effective nuclear attraction on outer energy level electrons decrease. e.g. The 1st ionization energy of Magnesium is 900 kJmole-1 while that of Calcium is 590 kJmole-1 .This is because atomic radius increase and thus effective nuclear attraction on outer energy level electrons decrease down the group from magnesium to calcium.
It requires therefore less energy to donate/lose outer electron in calcium than in magnesium.
The minimum amount of energy required to remove a second electron from an ion of an element in its gaseous state is called the 2nd ionization energy.
The 2nd ionization energy is always higher /bigger than the 1st ionization energy.
This is because once an electron is donated /lost form an atom, the overall effective nuclear attraction on the remaining electrons/energy level increase. Removing a second electron from the ion require therefore more energy than the first electron.
The atomic radius of alkali metals is higher/bigger than that of alkaline earth metals. This is because across/along the period from left to right there is an increase in nuclear charge from additional number of protons and still additional number of electrons entering the same energy level. Increase in nuclear charge increases the effective nuclear attraction on the outer energy level which pulls it closer to the nucleus. e.g.
Atomic radius of Sodium (0.157nM) is higher than that of Magnesium (0.137nM). This is because Magnesium has more effective nuclear attraction on the outer energy level than Sodium hence pulls outer energy level more nearer to its nucleus.
Physical properties
Soft/Easy to cut: Alkaline earth metals are not soft and easy to cut with a knife like alkali metals. This is because of the decrease in atomic radius of corresponding alkaline earth metal, increases the strength of metallic bond and the packing of the metallic structure. Alkaline earth metals are:
- ductile(able to form wire/thin long rods)
- malleable(able to be hammered into sheet/long thin plates)
- have high tensile strength(able to be coiled without breaking/ not brittle/withstand stress)
Appearance: Alkali earth metals have a shiny grey metallic luster when their surface is freshly polished /scrubbed. The surface slowly tarnishes on exposure to air. This is because the metal surface slowly undergoes oxidation to form an oxide. This oxide layer should be removed before using the alkaline earth metals.
Melting and boiling points: Alkaline earth metals have a relatively high melting/ boiling point than alkali metals. This is because alkali metals use only one delocalized electron to form a weaker metallic bond/structure. Alkaline earth metals use two delocalized electrons to form a stronger metallic bond /structure.
Themelting and boiling points decrease down the group as the atomic radius/size increase reducing the strength of metallic bond and packing of the metallic structure. e.g.
Beryllium has a melting point of 1280oC. Magnesium has a melting point of 650oC.Beryllium has a smaller atomic radius/size than magnesium .The strength of metallic bond and packing of the metallic structure is thus stronger in beryllium.
Electrical/thermal conductivity: Alkaline earth metals are good thermal and electrical conductors. The two delocalized valence electrons move randomly within the metallic structure.
Electrical conductivity increase down the group as the atomic radius/size increase making the delocalized outer electrons less attracted to nucleus. Alkaline earth metals are better thermal and electrical conductors than alkali metals because they have more/two outer delocalized electrons. e.g.
Magnesium is a better conductor than sodium because it has more/two delocalized electrons than sodium. The more delocalized electrons the better the electrical conductor.
Calcium is a better conductor than magnesium.
Calcium has bigger/larger atomic radius than magnesium because the delocalized electrons are less attracted to the nucleus of calcium and thus more free /mobile and thus better the electrical conductor
Summary of some physical properties of the 1st three alkaline earth metals
| Alkaline earth metal | Appearance | Ease of cutting | Melting point (oC) | Boiling point (oC) | Conduct- ivity | 1st ionization energy | 2nd ionization energy |
| Beryllium | Shiny grey | Not easy | 1280 | 3450 | Good | 900 | 1800 |
| Magnesium | Shiny grey | Not Easy | 650 | 1110 | Good | 736 | 1450 |
| calcium | Shiny grey | Not easy | 850 | 1140 | Good | 590 | 970 |
Chemical properties
- Reaction with air/oxygen
On exposure to air, the surface of alkaline earth metals is slowly oxidized to its oxide on prolonged exposure to air.
Example
On exposure to air, the surface of magnesium ribbon is oxidized to form a thin film of Magnesium oxide
. 2Mg(s) + O2(g) -> 2MgO(s)
(ii) Burning in air/oxygen
Experiment
Hold a about 2cm length of Magnesium ribbon on a Bunsen flame. Stop heating when it catches fire/start burning.
Caution: Do not look directly at the flame
Put the products of burning into 100cm3 beaker. Add about 5cm3 of distilled water. Swirl. Test the mixture using litmus papers.
Repeat with Calcium
Observations
-Magnesium burns with a bright blindening flame
-White solid /ash produced
-Solid dissolves in water to form a colourless solution
-Blue litmus paper remain blue
-Red litmus paper turns blue
-colourless gas with pungent smell of urine
Explanation
Magnesium burns in air with a bright blindening flame to form a mixture of Magnesium oxide and Magnesium nitride.
2Mg (s) + O2 (g) -> 2MgO(s)
3Mg (s) + N2 (g) -> Mg3N2 (s)
Magnesium oxide dissolves in water to form magnesium hydroxide.
MgO(s) + H2O (l) -> Mg (OH)2(aq)
Magnesium nitride dissolves in water to form magnesium hydroxide and produce ammonia gas.
Mg3N2 (s) + 6H2O (l) -> 3Mg (OH)2(aq) + 2NH3 (g)
Magnesium hydroxide and ammonia are weakly alkaline with pH 8/9/10/11 and turns red litmus paper blue.
Calcium burns in air with faint orange/red flame to form a mixture of both Calcium oxide and calcium nitride.
2Ca (s) + O2 (g) -> 2CaO(s)
3Ca (s) + N2 (g) -> Ca3N2 (s)
Calcium oxide dissolves in water to form calcium hydroxide.
CaO(s) + H2O (l) -> Ca (OH)2(aq)
Calcium nitride dissolves in water to form calcium hydroxide and produce ammonia gas.
Ca3N2 (s) + 6H2O (l) -> 3Ca (OH)2(aq) + 2NH3 (g)
Calcium hydroxide is also weakly alkaline solution with pH 8/9/10/11 and turns red litmus paper blue.
- Reaction with water
Experiment
Measure 50 cm3 of distilled water into a beaker.
Scrub/polish with sand paper 1cm length of Magnesium ribbon
Place it in the water. Test the product-mixture with blue and red litmus papers.
Repeat with Calcium metal.
Observations
-Surface of magnesium covered by bubbles of colourless gas.
-Colourless solution formed.
-Effervescence/bubbles/fizzing takes place in Calcium.
-Red litmus paper turns blue.
-Blue litmus paper remains blue.
Explanations
Magnesium slowly reacts with cold water to form Magnesium hydroxide and bubbles of Hydrogen gas that stick on the surface of the ribbon.
Mg(s) + 2H2O (l) -> Mg(OH)2(aq) + H2 (g)
Calcium moderately reacts with cold water to form Calcium hydroxide and produce a steady stream of Hydrogen gas.
Ca(s) + 2H2O (l) -> Ca (OH)2(aq) + H2 (g)
- Reaction with water vapour/steam
Experiment
Put some cotton wool soaked in water/wet sand in a long boiling tube.
Coil a well polished magnesium ribbon into the boiling tube.
Ensure the coil touches the side of the boiling tube. Heat the cotton wool/sand slightly then strongly heat the Magnesium ribbon.
Set up of apparatus
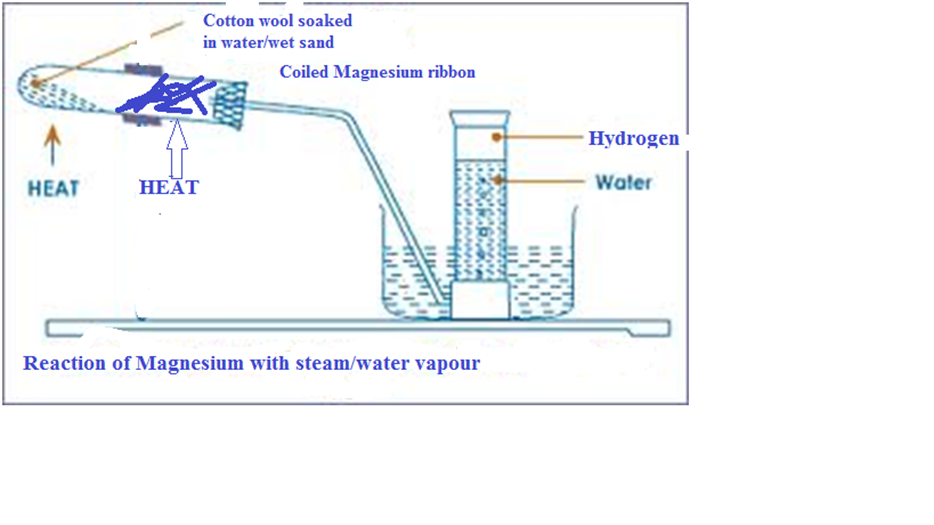
Observations
-Magnesium glows red hot then burns with a blindening flame.
-Magnesium continues to glow/burning even without more heating.
-White solid/residue.
-colourless gas collected over water.
Explanation
On heating wet sand, steam is generated which drives out the air that would otherwise react with /oxidize the ribbon.
Magnesium burns in steam/water vapour generating enough heat that ensures the reaction goes to completion even without further heating. White Magnesium oxide is formed and hydrogen gas is evolved.
To prevent suck back, the delivery tube should be removed from the water before heating is stopped at the end of the experiment.
Mg(s) + H2O (l) -> MgO(s) + H2 (g)
- Reaction with chlorine gas.
Experiment
Lower slowly burning magnesium ribbon/shavings into a gas jar containing Chlorine gas. Repeat with a hot piece of calcium metal.
Observation
-Magnesium continues to burn in chlorine with a bright blindening flame.
-Calcium continues to burn for a short time.
-White solid formed.
-Pale green colour of chlorine fades.
Explanation
Magnesium continues to burn in chlorine gas forming white magnesium oxide solid.
Mg(s) + Cl2 (g) -> MgCl2 (s)
Calcium burns slightly in chlorine gas to form white calcium oxide solid. Calcium oxide formed coat unreacted Calcium stopping further reaction
Ca(s) + Cl2 (g) -> CaCl2 (s)
- Reaction with dilute acids.
Experiment
Place about 4.0cm3 of 0.1M dilute sulphuric (VI) acid into a test tube. Add about 1.0cm length of magnesium ribbon into the test tube. Cover the mouth of the test tube using a thumb. Release the gas and test the gas using a burning splint.
Repeat with about 4.0cm3 of 0.1M dilute hydrochloric/nitric (V) acid.
Repeat with 0.1g of Calcium in a beaker with all the above acid
Caution: Keep distance when using calcium
Observation
-Effervescence/fizzing/bubbles with dilute sulphuric (VI) and nitric (V) acids
-Little Effervescence/fizzing/bubbles with calcium and dilute sulphuric (VI) acid.
-Colourless gas produced that extinguishes a burning splint with an explosion/ “pop” sound.
-No gas is produced with Nitric (V) acid.
-Colourless solution is formed.
Explanation
Dilute acids react with alkaline earth metals to form a salt and produce hydrogen gas.
Nitric (V) acid is a strong oxidizing agent. It quickly oxidizes the hydrogen produced to water.
Calcium is very reactive with dilute acids and thus a very small piece of very dilute acid should be used.
Chemical equations
Mg(s) + H2SO4 (aq) -> MgSO4 (aq) + H2 (g)
Mg(s) + 2HNO3 (aq) -> Mg(NO3)2(aq) + H2 (g)
Mg(s) + 2HCl (aq) -> MgCl2(aq) + H2 (g)
Ca(s) + H2SO4 (aq) -> CaSO4(s) + H2 (g)
(insoluble CaSO4(s) coat/cover Ca(s))
Ca(s) + 2HNO3 (aq) -> Ca(NO3)2(aq) + H2 (g)
Ca(s) + 2HCl (aq) -> CaCl2(aq) + H2 (g)
Ba(s) + H2SO4 (aq) -> BaSO4(s) + H2 (g)
(insoluble BaSO4(s) coat/cover Ba(s))
Ba(s) + 2HNO3 (aq) -> Ba(NO3)2(aq) + H2 (g)
Ba(s) + 2HCl (aq) -> BaCl2(aq) + H2 (g)
The table below shows some compounds of some alkaline earth metals
| Beryllium | Magnesium | Calcium | Barium | |
| Hydroxide | Be(OH)2 | Mg(OH)2 | Ca(OH)2 | Ba(OH)2 |
| Oxide | BeO | MgO | CaO | BaO |
| Sulphide | – | MgS | CaS | BaS |
| Chloride | BeCl2 | MgCl2 | CaCl2 | BaCl2 |
| Carbonate | BeCO3 | MgCO3 | CaCO3 | BaCO3 |
| Nitrate(V) | Be(NO3)2 | Mg(NO3)2 | Ca(NO3)2 | Ba(NO3)2 |
| Sulphate(VI) | BeSO4 | MgSO4 | CaSO4 | BaSO4 |
| Sulphate(IV) | – | – | CaSO3 | BaSO3 |
| Hydrogen carbonate | – | Mg(HCO3)2 | Ca(HCO3)2 | – |
| Hydrogen sulphate(VI) | – | Mg(HSO4)2 | Ca(HSO4)2 | – |
Some uses of alkaline earth metals include:
(i)Magnesium hydroxide is a non-toxic/poisonous mild base used as an anti acid medicine to relieve stomach acidity.
(ii) Making duralumin. Duralumin is an alloy of Magnesium and aluminium used for making aeroplane bodies because it is light.
(iii) Making plaster of Paris-Calcium sulphate (VI) is used in hospitals to set a fractures bone.
(iii)Making cement-Calcium carbonate is mixed with clay and sand then heated to form cement for construction/building.
(iv)Raise soil pH-Quicklime/calcium oxide is added to acidic soils to neutralize and raise the soil pH in agricultural farms.
(v) As nitrogenous fertilizer-Calcium nitrate (V) is used as an agricultural fertilizer because plants require calcium for proper growth.
(vi)In the blast furnace-Limestone is added to the blast furnace to produce more reducing agent and remove slag in the blast furnace for extraction of Iron.
(c)Group VII elements: Halogens
Group VII elements are called Halogens. They are all non metals. They include:
| Element | Symbol | Atomic number | Electronicc configuration | Charge of ion | Valency | State at Room Temperature |
| Fluorine Chlorine Bromine Iodine Astatine | F Cl Br I At | 9 17 35 53 85 | 2:7 2:8:7 2:8:18:7 2:8:18:18:7 2:8:18:32:18:7 | F– Cl– Br– I– At– | 1 1 1 1 1 | Pale yellow gas Pale green gas Red liquid Grey Solid Radioactive |
All halogen atoms have seven electrons in the outer energy level. They acquire/gain one electron in the outer energy level to be stable. They therefore are therefore monovalent .They exist in oxidation state X–
The number of energy levels increases down the group from Fluorine to Astatine. The more the number of energy levels the bigger/larger the atomic size. e.g.
The atomic size/radius of Chlorine is bigger/larger than that of Fluorine because Chlorine has more/3 energy levels than Fluorine (2 energy levels).
Atomic radius and ionic radius of Halogens increase down the group as the number of energy levels increases.
The atomic radius of Halogens is smaller than the ionic radius. This is because they react by gaining/acquiring extra one electron in the outer energy level. The effective nuclear attraction on the more/extra electrons decreases. The incoming extra electron is also repelled causing the outer energy level to expand to reduce the repulsion and accommodate more electrons.
Table showing the atomic and ionic radius of four Halogens
| Element | Symbol | Atomic number | Atomic radius(nM) | Ionic radius(nM) |
| Fluorine | F | 9 | 0.064 | 0.136 |
| Chlorine | Cl | 17 | 0.099 | 0.181 |
| Bromine | Br | 35 | 0.114 | 0.195 |
| Iodine | I | 53 | 0.133 | 0.216 |
The atomic radius of Chlorine is 0.099nM .The ionic radius of Cl– is 0.181nM. This is because Chlorine atom/molecule reacts by gaining/acquiring extra one electron. The more/extra electrons/energy level experience less effective nuclear attraction /pull towards the nucleus. The outer energy level expand/increase to reduce the repulsion of the existing and incoming gained /acquired electrons.
Electronegativity
The ease of gaining/acquiring extra electrons is called electronegativity. All halogens are electronegative. Electronegativity decreases as atomic radius increase. This is because the effective nuclear attraction on outer electrons decreases with increase in atomic radius.
The outer electrons experience less nuclear attraction and thus ease of gaining/acquiring extra electrons decrease.
It is measured using Pauling’s scale.
Where Fluorine with Pauling scale 4.0 is the most electronegative element and thus the highest tendency to acquire/gain extra electron.
Table showing the electronegativity of the halogens
| Halogen | F | Cl | Br | I | At |
| Electronegativity (Pauling scale) | 4.0 | 3.0 | 2.8 | 2.5 | 2.2 |
The electronegativity of the halogens decrease down the group from fluorine to Astatine. This is because atomic radius increases down the group and thus decrease electron – attracting power down the group from fluorine to astatine.
Fluorine is the most electronegative element in the periodic table because it has the small atomic radius.
Electron affinity
The minimum amount of energy required to gain/acquire an extra electron by an atom of element in its gaseous state is called 1st electron affinity. The SI unit of electron affinity is kilojoules per mole/kJmole-1. Electron affinity depends on atomic radius. The higher the atomic radius, the less effective the nuclear attraction on outer energy level electrons and thus the lower the electron affinity. For halogens the 1st electron affinity decrease down the group as the atomic radius increase and the effective nuclear attraction on outer energy level electrons decrease. Due to its small size/atomic radius Fluorine shows exceptionally low electron affinity. This is because a lot of energy is required to overcome the high repulsion of the existing and incoming electrons.
Table showing the election affinity of halogens for the process
X + e -> X–
| Halogen | F | Cl | Br | I |
| Electron affinity kJmole-1 | -333 | -364 | -342 | -295 |
The higher the electron affinity the more stable theion.i.e
Cl– is a more stable ion than Br– because it has a more negative / exothermic electron affinity than Br–
Electron affinity is different from:
(i) Ionization energy.
Ionization energy is the energy required to lose/donate an electron in an atom of an element in its gaseous state while electron affinity is the energy required to gain/acquire extra electron by an atom of an element in its gaseous state.
(ii) Electronegativity.
-Electron affinity is the energy required to gain an electron in an atom of an element in gaseous state. It involves the process:
X(g) + e -> X–(g)
Electronegativity is the ease/tendency of gaining/ acquiring electrons by an element during chemical reactions.
It does not involve use of energy but theoretical arbitrary Pauling’ scale of measurements.
Physical properties
State at room temperature
Fluorine and Chlorine are gases, Bromine is a liquid and Iodine is a solid. Astatine is radioactive.
All halogens exist as diatomic molecules bonded by strong covalent bond. Each molecule is joined to the other by weak intermolecular forces/ Van-der-waals forces.
Melting/Boiling point
The strength of intermolecular/Van-der-waals forces of attraction increase with increase in molecular size/atomic radius.
Iodine has therefore the largest atomic radius and thus strongest intermolecular forces to make it a solid.
Iodine sublimes when heated to form (caution: highly toxic/poisonous) purple vapour.
This is because Iodine molecules are held together by weak van-der-waals/intermolecular forces which require little heat energy to break.
Electrical conductivity
All Halogens are poor conductors of electricity because they have no free delocalized electrons.
Solubility in polar and non-polar solvents
All halogens are soluble in water (polar solvent).
When a boiling tube containing either chlorine gas or bromine vapour is separately inverted in a beaker containing distilled water and tetrachloromethane (non-polar solvent), the level of solution in boiling tube rises in both water and tetrachloromethane.
This is because halogen are soluble in both polar and non-polar solvents. Solubility of halogens in water/polar solvents decrease down the group. Solubility of halogens in non-polar solvent increases down the group.
The level of water in chlorine is higher than in bromine and the level of tetrachloromethane in chlorine is lower than in bromine.
Caution: Tetrachloromethane , Bromine vapour and Chlorine gas are all highly toxic/poisonous.
Table showing the physical properties of Halogens
| Halogen | Formula of molecule | Electrical conductivity | Solubility in water | Melting point(oC) | Boiling point(oC) |
| Fluorine | F2 | Poor | Insoluble/soluble in tetrachloromethane | -238 | -188 |
| Chlorine | Cl2 | Poor | Insoluble/soluble in tetrachloromethane | -101 | -35 |
| Bromine | Br2 | Poor | Insoluble/soluble in tetrachloromethane | 7 | 59 |
| Iodine | I2 | Poor | Insoluble/soluble in tetrachloromethane | 114 | sublimes |
Chemical properties
(i)Displacement
Experiment
Place separately in test tubes about 5cm3 of sodium chloride, Sodium bromide and Sodium iodide solutions.
Add 5 drops of chlorine water to each test tube:
Repeat with 5 drops of bromine water instead of chlorine water
Observation
Using Chlorine water
-Yellow colour of chlorine water fades in all test tubes except with sodium chloride.
-Coloured Solution formed.
Using Bromine water
-Yellow colour of bromine water fades in test tubes containing sodium iodide.
-Coloured Solution formed.
Explanation
The halogens displace each other from their solution. The more electronegative displace the less electronegative from their solution.
Chlorine is more electronegative than bromine and iodine.
On adding chlorine water, bromine and Iodine are displaced from their solutions by chlorine.
Bromine is more electronegative than iodide but less 6than chlorine.
On adding Bromine water, iodine is displaced from its solution but not chlorine.
Table showing the displacement of the halogens
(V) means there is displacement (x ) means there is no displacement
| Halogen ion in solution Halogen | F– | Cl– | Br– | I– |
| F2 | X | |||
| Cl2 | X | X | ||
| Br2 | X | X | X | |
| I2 | X | X | X | X |
Chemical /ionic equations
With Fluorine
F2(g) + 2NaCl–(aq) -> 2NaF(aq) + Cl2(aq)
F2(g) + 2Cl–(aq) -> 2F–(aq) + Cl2(aq)
F2(g) + 2NaBr–(aq) -> 2NaF(aq) + Br2(aq)
F2(g) + 2Br–(aq) -> 2F–(aq) + Br2(aq)
F2(g) + 2NaI–(aq) -> 2NaF(aq) + I2(aq)
F2(g) + 2I–(aq) -> 2F–(aq) + I2(aq)
With chlorine
Cl2(g) + 2NaCl–(aq) -> 2NaCl(aq) + Br2(aq)
Cl2(g) + 2Br–(aq) -> 2Cl–(aq) + Br2(aq)
Cl2(g) + 2NaI–(aq) -> 2NaCl(aq) + I2(aq)
Cl2(g) + 2I–(aq) -> 2Cl–(aq) + I2(aq)
With Bromine
Br2(g) + 2NaI–(aq) -> 2NaBr(aq) + I2(aq)
Br2(g) + 2I–(aq) -> 2Br–(aq) + I2(aq)
Uses of halogens
- Florine – manufacture of P.T.F.E (Poly tetra fluoroethene) synthetic fiber.
- Reduce tooth decay when added in small amounts/quantities in tooth paste.
NB –large small quantities of fluorine /fluoride ions in water cause browning of teeth/flourosis.
- Hydrogen fluoride is used to engrave words /pictures in glass.
- Bromine – Silver bromide is used to make light sensitive photographic paper/films.
- Iodide – Iodine dissolved in alcohol is used as medicine to kill bacteria in skin cuts. It is called tincture of iodine.
The table below to show some compounds of halogens
| Element Halogen | H | Na | Mg | Al | Si | C | P |
| F | HF | NaF | MgH2 | AlF3 | SiF4 | CF4 | PF3 |
| Cl | HCl | NaCl | MgCl | AlCl3 | SiCl3 | CCl4 | PCl3 |
| Br | HBr | NaBr | MgBr2 | AlBr3 | SiBr4 | CBr4 | PBr3 |
| I | Hl | Nal | Mgl2 | All3 | SiI4 | Cl2 | PBr3 |
- Below is the table showing the bond energy of four halogens.
Bond Bond energy k J mole-1
Cl-Cl 242
Br-Br 193
I-I 151
- What do you understand by the term “bond energy”
Bond energy is the energy required to break/ form one mole of chemical bond
- Explain the trend in bond Energy of the halogens above:
–Decrease down the group from chlorine to Iodine
-Atomic radius increase down the group decreasing the energy required to break the covalent bonds between the larger atom with reduced effective nuclear @ charge an outer energy level that take part in bonding.
(c)Group VIII elements: Noble gases
Group VIII elements are called Noble gases. They are all non metals. Noble gases occupy about 1.0% of the atmosphere as colourless gaseous mixture. Argon is the most abundant with 0.9%.
They exist as monatomic molecules with very weak van-der-waals /intermolecular forces holding the molecules.
They include:
| Element | Symbol | Atomic number | Electron structure | State at room temperature |
| Helium | He | 2 | 2: | Colourless gas |
| Neon | Ne | 10 | 2:8 | Colourless gas |
| Argon | Ar | 18 | 2:8:8 | Colourless gas |
| Krypton | Kr | 36 | 2:8:18:8 | Colourless gas |
| Xenon | Xe | 54 | 2:8:18:18:8 | Colourless gas |
| Radon | Rn | 86 | 2:8:18:32:18:8 | Radioctive |
All noble gas atoms have a stable duplet(two electrons in the 1st energy level) or octet(eight electrons in other outer energy level)in the outer energy level. They therefore do not acquire/gain extra electron in the outer energy level or donate/lose. They therefore are therefore zerovalent .
The number of energy levels increases down the group from Helium to Randon. The more the number of energy levels the bigger/larger the atomic size/radius. e.g.
The atomic size/radius of Argon is bigger/larger than that of Neon because Argon has more/3 energy levels than Neon (2 energy levels).
Atomic radius noble gases increase down the group as the number of energy levels increases.
The effective nuclear attraction on the outer electrons thus decrease down the group.
The noble gases are generally unreactive because the outer energy level has the stable octet/duplet. The stable octet/duplet in noble gas atoms lead to comparatively very high 1st ionization energy. This is because losing /donating an electron from the stable atom require a lot of energy to lose/donate and make it unstable.
As atomic radius increase down the group and the 1st ionization energy decrease, very electronegative elements like Oxygen and Fluorine are able to react and bond with lower members of the noble gases.e.g
Xenon reacts with Fluorine to form a covalent compound XeF6.This is because the outer electrons/energy level if Xenon is far from the nucleus and thus experience less effective nuclear attraction.
Noble gases have low melting and boiling points. This is because they exist as monatomic molecules joined by very weak intermolecular/van-der-waals forces that require very little energy to weaken and form liquid and break to form a gas.
The intermolecular/van-der-waals forces increase down the group as the atomic radius/size increase from Helium to Radon. The melting and boiling points thus increase also down the group.
Noble gases are insoluble in water and are poor conductors of electricity.
| Element | Formula of molecule | Electrical conductivity | Solubility in water | Atomic radius(nM) | 1st ionization energy | Melting point(0C) | Boiling point(0C) |
| Helium | He | Poor | Insoluble | 0.128 | 2372 | -270 | -269 |
| Neon | Ne | Poor | Insoluble | 0.160 | 2080 | -249 | -246 |
| Argon | Ar | Poor | Insoluble | 0.192 | 1520 | -189 | -186 |
| Krypton | Kr | Poor | Insoluble | 0.197 | 1350 | -157 | -152 |
| Xenon | Xe | Poor | Insoluble | 0.217 | 1170 | -112 | -108 |
| Radon | Rn | Poor | Insoluble | 0.221 | 1134 | -104 | -93 |
Uses of noble gases
Argon is used in light bulbs to provide an inert environment to prevent oxidation of the bulb filament
Argon is used in arch welding as an insulator.
Neon is used in street and advertisement light
Helium is mixed with Oxygen during deep sea diving and mountaineering.
Helium is used in weather balloon for meteorological research instead of Hydrogen because it is unreactive/inert.Hydrogen when impure can ignite with an explosion.
Helium is used in making thermometers for measuring very low temperatures.
