The atom is the smallest particle of an element that take part in a chemical reaction. The atom is made up of three subatomic particles:
(i)Protons
(ii)Electrons
(iii)Neutrons
(i)Protons
1. The proton is positively charged
2. Is found in the centre of an atom called nucleus
3. It has a relative mass 1
4. The number of protons in a atom of an element is its Atomic number
(ii)Electrons
1. The Electrons is negatively charged
2. Is found in fixed regions surrounding the centre of an atom called energy levels/orbitals.
3. It has a relative mass 1/1840
4. The number of protons and electrons in a atom of an element is always equal
(iii)Neutrons
1. The Neutron is neither positively nor negatively charged thus neutral.
2. Like protons it is found in the centre of an atom called nucleus
3. It has a relative mass 1
4. The number of protons and neutrons in a atom of an element is its Mass number
Diagram showing the relative positions of protons, electrons and neutrons in an atom of an element
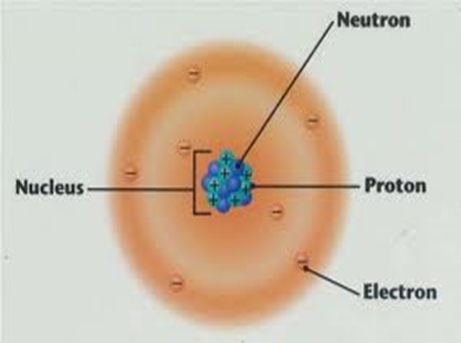
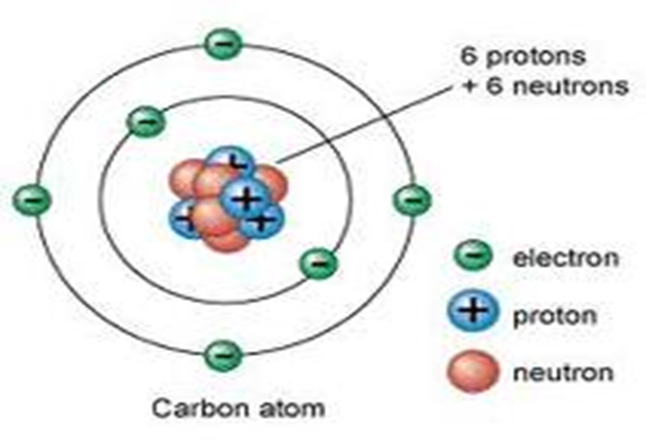
Diagram showing the relative positions of protons, electrons and neutrons in an atom of Carbon
The table below show atomic structure of the 1st twenty elements.
Element | Symbol | Protons | Electrons | Neutrons | Atomic number | Mass number |
| Hydrogen | H | 1 | 1 | 0 | 1 | 1 |
| Helium | He | 2 | 2 | 2 | 2 | 4 |
| Lithium | Li | 3 | 3 | 4 | 3 | 7 |
| Beryllium | Be | 4 | 4 | 5 | 4 | 9 |
| Boron | B | 5 | 5 | 6 | 5 | 11 |
| Carbon | C | 6 | 6 | 6 | 6 | 12 |
| Nitrogen | N | 7 | 7 | 7 | 7 | 14 |
| Oxygen | O | 8 | 8 | 8 | 8 | 16 |
| Fluorine | F | 9 | 9 | 10 | 9 | 19 |
| Neon | Ne | 10 | 10 | 10 | 10 | 20 |
| Sodium | Na | 11 | 11 | 12 | 11 | 23 |
| Magnesium | Mg | 12 | 12 | 12 | 12 | 24 |
| Aluminium | Al | 13 | 13 | 14 | 13 | 27 |
| Silicon | Si | 14 | 14 | 14 | 14 | 28 |
| Phosphorus | P | 15 | 15 | 16 | 15 | 31 |
| Sulphur | S | 16 | 16 | 16 | 16 | 32 |
| Chlorine | Cl | 17 | 17 | 18 | 17 | 35 |
| Argon | Ar | 18 | 18 | 22 | 18 | 40 |
| Potassium | K | 19 | 19 | 20 | 19 | 39 |
| Calcium | Ca | 20 | 20 | 20 | 20 | 40 |
Most atoms of elements exist as isotopes.
Isotopes are atoms of the same element, having the same number of protons/atomic number but different number of neutrons/mass number.
By convention, isotopes are written with the mass number as superscript and the atomic number as subscript to the left of the chemical symbol of the element. i.e.
mass number
atomic number m n X symbol of element
Below is the conventional method of writing the 1st twenty elements showing the mass numbers and atomic numbers:
11H 42He 73Li 94Be 115B 126C
147N 168O 199F 2010Ne 2311Na 2412Mg
2713Al 2814Si 3115P 3216S 3517Cl 4018Ar
3919K 4020C
The table below shows some common natural isotopes of some elements
| Element | Isotopes | Protons | Electrons | Neutrons | Atomic number | Mass number |
| Hydrogen | 11H 21H(deuterium) 31H(Tritium) | 1 1 1 | 1 1 1 | 0 2 3 | 1 1 1 | 1 2 3 |
| Chlorine | 3517Cl 3717Cl | 17 17 | 17 17 | 18 20 | 17 17 | 35 37 |
| Potassium | 3919K 4019K 4119K | 19 19 19 | 19 19 19 | 20 21 22 | 19 19 19 | 39 40 41 |
| Oxygen | 168O 188O | 8 8 | 8 8 | 8 10 | 8 8 | 16 18 |
| Uranium | 23592U 23892U | 92 92 | 92 92 | 143 146 | 92 92 | 235 238 |
| Neon | 2210Ne 2010Ne 2110Ne | 10 10 10 | 10 10 10 | 12 10 11 | 10 10 10 | 22 20 21 |
The mass of an average atom is very small (10-22 g).Masses of atoms are therefore expressed in relation to a chosen element.
The atom recommended is 12C isotope whose mass is arbitrarily assigned as 12.000 atomic mass units(a.m.u) .
All other atoms are compared to the mass of 12C isotope to give the relative at The relative atomic mass(RAM) is therefore defined as “the mass of average atom of an element compared to 1/12 an atom of 12C isotope whose mass is arbitrarily fixed as 12.000 atomic mass units(a.m.u) ” i.e;
RAM = mass of atom of an element
1/12 of one atom of 12C isotope
Accurate relative atomic masses (RAM) are got from the mass spectrometer. Mass spectrometer determines the isotopes of the element and their relative abundance/availability.
Using the relative abundances/availability of the isotopes, the relative atomic mass (RAM) can be determined /calculated as in the below examples.
- Chlorine occurs as 75% 3517Cl and 25% 3717Cl isotopes. Calculate the relative atomic mass of Chlorine.
Working
100 atoms of chlorine contains 75 atoms of 3517Cl isotopes
100 atoms of chlorine contains 75 atoms of 3717Cl isotopes
Therefore;
RAM of chlorine = ( 75/100 x 35) + 25/100 x 37 = 35.5
Note that:
Relative atomic mass has no units
More atoms of chlorine exist as 3517Cl(75%) than as 3717Cl(25%) therefore RAM is nearer to the more abundant isotope.
- Calculate the relative atomic mass of potassium given that it exist as;
93.1% 3919K , 0.01% 4019K , 6.89% 4119K ,
Working
100 atoms of potassium contains 93.1 atoms of 3919K isotopes
100 atoms of potassium contains 0.01 atoms of 4019K isotopes
100 atoms of potassium contains 6.89 atoms of 4119K isotopes
Therefore;
RAM of potassium = (93.1/100 x39) + (0.01/100 x 40) +(6.89 /100 x 39) Note that:
Relative atomic mass has no units
More atoms of potassium exist as 3919K (93.1%) therefore RAM is nearer to the more abundant 3919K isotope.
- Calculate the relative atomic mass of Neon given that it exist as;
90.92% 2010Ne , 0.26% 2110Ne , 8.82% 2210Ne,
Working
100 atoms of Neon contains 90.92 atoms of 2010Ne isotopes
100 atoms of Neon contains 0.26 atoms of 2110Ne isotopes
100 atoms of Neon contains 8.82 atoms of 2210 Ne isotopes Therefore;
RAM of Neon = (90.92/100 x20) + (0.26/100 x 21) +(8.82 /100 x 22)
Note that:
Relative atomic mass has no units
More atoms of Neon exist as 2010Ne (90.92%) therefore RAM is nearer to the more abundant 2010Ne isotope.
- Calculate the relative atomic mass of Argon given that it exist as;
90.92% 2010Ne , 0.26% 2110Ne , 8.82% 2210Ne,
NB
The relative atomic mass is a measure of the masses of atoms. The higher the relative atomic mass, the heavier the atom.
Electrons are found in energy levels/orbital.
An energy level is a fixed region around/surrounding the nucleus of an atom occupied by electrons of the same (potential) energy.
By convention energy levels are named 1,2,3… outwards from the region nearest to nucleus.
Each energy level is occupied by a fixed number of electrons:
The 1st energy level is occupied by a maximum of two electrons
The 2nd energy level is occupied by a maximum of eight electrons
The 3rd energy level is occupied by a maximum of eight electrons( or eighteen electrons if available)
The 4th energy level is occupied by a maximum of eight electrons( or eighteen or thirty two electrons if available)
This arrangement of electrons in an atom is called electron configuration / structure.
By convention theelectron configuration / structure of an atom of an element can be shown in form of a diagram using either cross(x) or dot(●) to
Practice examples drawing electronic configurations
a)11H has – in nucleus1proton and 0 neutrons
-1 electron in the 1st energy levels thus:
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Hydrogen is thus: 1:
b) 42He has – in nucleus 2 proton and 2 neutrons[s1]
-2 electron in the 1st energy levels thus:
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Helium is thus: 2:
c) 73Li has – in nucleus 3 proton and 4 neutrons
– 2 electron in the 1st energy levels
–1 electron in the 2nd energy levels thus
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Lithium is thus: 2:1
d) 94Be has – in nucleus 4 proton and 5 neutrons
– 2 electron in the 1st energy levels
–2 electron in the 2nd energy levels thus
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Beryllium is thus: 2:2
e) 115B has – in nucleus 5 proton and 6 neutrons
– 2 electron in the 1st energy levels
–3 electron in the 2nd energy levels thus
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Boron is thus: 2:3
f) 126C has – in nucleus 6 proton and 6 neutrons
– 2 electron in the 1st energy levels
–4 electron in the 2nd energy levels thus
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Carbon is thus: 2:4
g) 147N has – in nucleus 7 proton and 7 neutrons
– 2 electron in the 1st energy levels
–5 electron in the 2nd energy levels thus
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Nitrogen is thus: 2:5
h) 168O has – in nucleus 8 proton and 8 neutrons
– 2 electron in the 1st energy levels
–6 electron in the 2nd energy levels thus
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Oxygen is thus: 2:6
i) 199F has – in nucleus 9 proton and 10 neutrons
– 2 electron in the 1st energy levels
–7 electron in the 2nd energy levels thus
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Fluorine is thus: 2:7
i) 2010Ne has – in nucleus 10 proton and 10 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels thus
Nucleus
Energy levels
Electrons (represented by cross(x)
Electronic structure of Neon is thus: 2:8
j) 2311Na has – in nucleus 11 proton and 12 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–1 electron in the 3rd energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Sodium is thus: 2:8:1
k) 2412Mg has – in nucleus 12 proton and 12 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–2 electron in the 3rd energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Magnesium is thus: 2:8:2
l) 2713Al has – in nucleus 13 proton and 14 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–3 electron in the 3rd energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Aluminium is thus: 2:8:3
m) 2814Si has – in nucleus 14 proton and 14 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–4 electron in the 3rd energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Silicon is thus: 2:8:4
n) 3115P has – in nucleus 14 proton and 15 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–5 electron in the 3rd energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Phosphorus is thus: 2:8:5
o) 3216S has – in nucleus 16 proton and 16 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–6 electron in the 3rd energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Sulphur is thus: 2:8:6
p) 3517Cl has – in nucleus 18 proton and 17 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–7 electron in the 3rd energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Chlorine is thus: 2:8:7
p) 4018Ar has – in nucleus 22 proton and 18 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–8 electron in the 3rd energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Argon is thus: 2:8:8
q) 3919K has – in nucleus 20 proton and 19 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–8 electron in the 3rd energy levels
–1 electron in the 4th energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Potassium is thus: 2:8:8:1
r) 4020Ca has – in nucleus 20 proton and 20 neutrons
– 2 electron in the 1st energy levels
–8 electron in the 2nd energy levels
–8 electron in the 3rd energy levels
–2 electron in the 4th energy levels thus
Nucleus
Energy levels
Electrons (represented by dot (.)
Electronic structure of Calcium is thus: 2:8:8:2
