- CHEMICAL BONDING
A chemical bond is formed when atoms of the same or different elements share, gain, donate or delocalize their outer energy level electrons to combine during chemical reactions in order to be stable.
Atoms have equal number of negatively charged electrons in the energy levels and positively charged protons in the nucleus.
Atoms are chemically stable if they have filled outer energy level. An energy level is full if it has duplet (2) or octet (8) state in outer energy level.
Noble gases have duplet /octet. All other atoms try to be like noble gases through chemical reactions and forming molecules.
Only electrons in the outer energy level take part in formation of a chemical bond. There are three main types of chemical bonds formed by atoms:
(i) Covalent bond
(ii) ionic/electrovalent bond
(iii) Metallic bond
(i)COVALENT BOND
A covalent bond is formed when atoms of the same or different element share some or all the outer energy level electrons to combine during chemical reactions in order to attain duplet or octet.
A shared pair of electrons is attracted by the nucleus (protons) of the two atoms sharing.
Covalent bonds are mainly formed by non-metals to form molecules. A molecule is a group of atoms of the same or different elements held together by a covalent bond. The number of atoms making a molecule is called atomicity. Noble gases are monatomic because they are stable and thus do not bond with each other or other atoms. Most other gases are diatomic
The more the number of electrons shared, the stronger the covalent bond.
A pair of electrons that do not take part in the formation of a covalent bond is called a lone pair of electrons.
Mathematically, the number of electrons to be shared by an atom is equal to the number of electrons remaining for the atom to be stable/attain duplet/octet /have maximum electrons in outer energy level.
The following diagrams illustrate the formation of covalent bonds:
a) Hydrogen molecule is made up of two hydrogen atoms in the outer energy level each requiring one electron to have a stable duplet.
To show the formation of covalent bonding in the molecule then the following data/information is required;
Symbol of atom/element taking part in bonding H H
Number of protons/electrons 1 1
Electron configuration/structure 1: 1:
Number of electron in outer energy level 1 1
Number of electrons remaining to be stable/shared 1 1
Number of electrons not shared (lone pairs) 0 0
Note:
After bonding the following intermolecular forces exist:
(i) The attraction of the shared electrons by both nucleus /protons of the atoms
(ii) The repulsion of the nucleus of one atom on the other.
(iii) Balance of the attraction and repulsion is maintained inside/intermolecular/within the molecule as follows;
b) Fluorine, chlorine, bromine and iodine molecules are made up also of two atoms sharing the outer energy level electrons to have a stable octet.
To show the formation of covalent bonding in the molecule then the following data/information is required:
(i) Fluorine
Symbol of atom/element taking part in bonding F F
Number of protons/electrons 9 9
Electron configuration/structure 2:7 2:7
Number of electron in outer energy level 7 7
Number of electrons remaining to be stable/shared 1 1
Number of outer electrons not shared (3-lone pairs) 6 6
(ii) Chlorine
Symbol of atom/element taking part in bonding Cl Cl
Number of protons/electrons 17 17
Electron configuration/structure 2:8:7 2:8:7
Number of electron in outer energy level 7 7
Number of electrons remaining to be stable/shared 1 1
Number of outer electrons not shared (3-lone pairs) 6 6
(iii) Bromine
Symbol of atom/element taking part in bonding Br Br
Number of protons/electrons 35 35
Electron configuration/structure 2:8:18:7 2:8:18:7
Number of electron in outer energy level 7 7
Number of electrons remaining to be stable/shared 1 1
Number of outer electrons not shared (3-lone pairs) 6 6
iv) Iodine
Symbol of atom/element taking part in bonding I I
Number of protons/electrons 53 53
Electron configuration/structure 2:8:18:18:7
Number of electron in outer energy level 7 7
Number of electrons remaining to be stable/shared 1 1
Number of outer electrons not shared (3-lone pairs) 6 6
iv) Iodine
Symbol of atom/element taking part in bonding I I
Number of protons/electrons 53 53
Electron configuration/structure 2:8:18:18:7
Number of electron in outer energy level 7 7
Number of electrons remaining to be stable/shared 1 1
Number of outer electrons not shared (3-lone pairs) 6 6
d) Nitrogen and phosphorus molecule is made up of two atoms sharing each three outer energy level electrons to have a stable octet as shown below;
(i) Nitrogen
Symbol of atom/element taking part in bonding N N
Number of protons/electrons 7 7
Electron configuration/structure 2:5 2:5
Number of electron in outer energy level 5 5
Number of electrons remaining to be stable/shared 3 3
Number of outer electrons not shared (3-lone pairs) 2 2
(ii) Phosphorus
Symbol of atom/element taking part in bonding P P
Number of protons/electrons 15 15
Electron configuration/structure 2:8:5 2:8:5
Number of electron in outer energy level 5 5
Number of electrons remaining to be stable/shared 3 3
Number of outer electrons not shared (3-lone pairs) 2 2
e) Water molecule is made up of hydrogen and oxygen. Hydrogen requires sharing one electron with oxygen to be stable/attain duplet. Oxygen requires to share two electrons to be stable/attain octet. Two hydrogen atoms share with one oxygen atom for both to be stable as shown below;
Symbol of atoms/elements taking part in bonding O H
Number of protons/electrons 8 1
Electron configuration/structure 2:6 1
Number of electron in outer energy level 6 1
Number of electrons remaining to be stable/shared 2 1
Number of electrons not shared (2-Oxygen lone pairs) 4 0
Diagram method 1
Diagram method 2
f) Ammonia molecule is made up of Hydrogen and Nitrogen. Hydrogen requires sharing one electron with Nitrogen to be stable/attain duplet. Nitrogen requires to share three electrons to be stable/attain octet. Three hydrogen atoms share with one nitrogen atom for both to be stable as shown below;
Symbol of atoms/elements taking part in bonding N H
Number of protons/electrons 7 1
Electron configuration/structure 2:5 1:
Number of electron in outer energy level 5 1
Number of electrons remaining to be stable/shared 3 1
Number of electrons not shared (1-Nitrogen lone pairs) 2 0
Diagram method 1
Diagram method 2
g) Carbon (IV) oxide molecule is made up of carbon and oxygen. Carbon requires to share four electrons with oxygen to be stable/attain octet. Oxygen requires to share two electrons to be stable/attain octet. Two oxygen atoms share with one carbon atom for both to be stable as shown below;
Symbol of atoms/elements taking part in bonding O C
Number of protons/electrons 8 6
Electron configuration/structure 2:6 2:4
Number of electron in outer energy level 6 4
Number of electrons remaining to be stable/shared 2 4
2-lone pairs from each Oxygen atom) 2 0
Diagram method 1
Diagram method 2
h) Methane molecule is made up of hydrogen and carbon. Hydrogen requires sharing one electron with carbon to be stable/attain duplet. Carbon requires sharing four electrons to be stable/attain octet. Four hydrogen atoms share with one carbon atom for both to be stable as shown below;
Symbol of atoms/elements taking part in bonding C H
Number of protons/electrons 6 1
Electron configuration/structure 2:4 1
Number of electron in outer energy level 4 1
Number of electrons remaining to be stable/shared 4 1
Number of electrons not shared ( No lone pairs) 0 0
Diagram method 1
Diagram method 2
i) Tetrachloromethane molecule is made up of chlorine and carbon. Chlorine requires sharing one electron with carbon to be stable/attain octet. Carbon requires sharing four electrons to be stable/attain octet. Four chlorine atoms share with one carbon atom for both to be stable as shown below;
Symbol of atoms/elements taking part in bonding C Cl
Number of protons/electrons 6 17
Electron configuration/structure 2:4 2:8:7
Number of electron in outer energy level 4 7
Number of electrons remaining to be stable/shared 4 1
3-lone pairs from each Chlorine atom (24 electrons) 0 6
Diagram method 1
Diagram method 2
j) Ethane molecule is made up of six hydrogen and two carbon atoms. Hydrogen requires to share one electron with carbon to be stable/attain duplet. Carbon requires to share four electrons to be stable/attain octet. Three hydrogen atoms share with one carbon atom while another three hydrogen atoms share with a different carbon atom. The two carbon atoms bond by sharing a pair of the remaining electrons as shown below;
Symbol of atoms/elements taking part in bonding C H
Number of protons/electrons 6 1
Electron configuration/structure 2:4 1
Number of electron in outer energy level 4 1
Number of electrons remaining to be stable/shared 4 1
Number of electrons not shared (No lone pairs) 0 0
Diagram method 1
Diagram method 2
k) Ethene molecule is made up of four hydrogen and two carbon atoms. Hydrogen requires to share one electron with carbon to be stable/attain duplet. Carbon requires to share four electrons to be stable/attain octet. Two hydrogen atoms share with one carbon atom while another two hydrogen atoms share with a different carbon atom. The two carbon atoms bond by sharing two pairs of the remaining electrons as shown below;
Symbol of atoms/elements taking part in bonding C H
Number of protons/electrons 6 1
Electron configuration/structure 2:4 1
Number of electron in outer energy level 4 1
Number of electrons remaining to be stable/shared 4 1
Number of electrons not shared (No lone pairs) 0 0
Diagram method 1
Diagram method 2
l) Ethyne molecule is made up of two hydrogen and two carbon atoms. Hydrogen requires to share one electron with carbon to be stable/attain duplet. Carbon requires to share four electrons to be stable/attain octet. One hydrogen atoms share with one carbon atom while another hydrogen atoms share with a different carbon atom. The two carbon atoms bond by sharing three pairs of the remaining electrons as shown below;
Symbol of atoms/elements taking part in bonding C H
Number of protons/electrons 6 1
Electron configuration/structure 2:4 1
Number of electron in outer energy level 4 1
Number of electrons remaining to be stable/shared 4 1
Number of electrons not shared (No lone pairs) 0 0
Diagram method 1
Diagram method 2
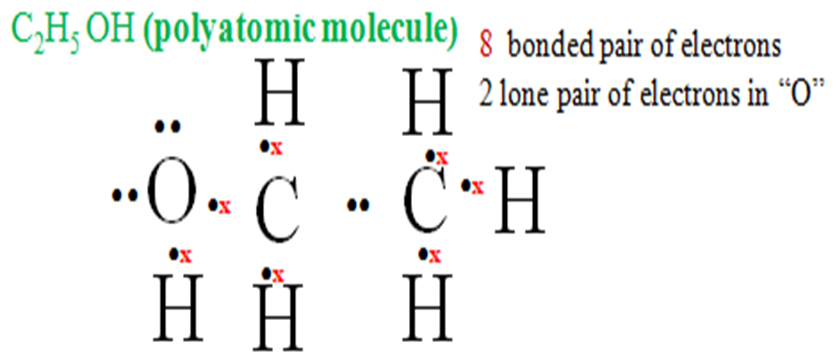
j) Ethanol molecule is made up of six hydrogen one Oxygen atom two carbon atoms.
Five Hydrogen atoms share their one electron each with carbon to be stable/attain duplet. One Hydrogen atoms share one electron with Oxygen for both to attain duplet/octet
Each Carbon uses four electrons to share with “O”and “H”attains octet/duplet.
NB: Oxygen has two lone pairs
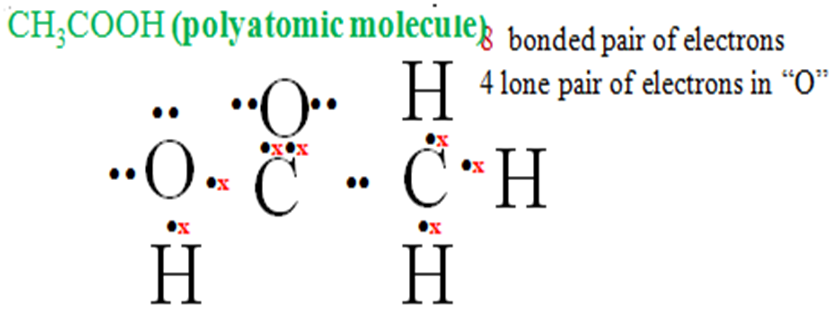
j) Ethanoic molecule is made up of four hydrogen two Oxygen atom two carbon atoms.
Three Hydrogen atoms share their one electron each with carbon to be stable/attain duplet. One Hydrogen atoms share one electron with Oxygen for both to attain duplet/octet
Each Carbon uses four electrons to share with “O”and “H”attains octet/duplet.
NB: Each Oxygen atom has two lone pairs
By convention (as a rule), a
(i) Single covalent bond made up of two shared (a pair) electrons is represented by a dash (—)
(ii) Double covalent bond made up of four shared (two pairs) electrons is represented by a double dash (==)
(iii) Triple covalent bond made up of six shared (three pairs) electrons is represented by a triple dash (==)
The representation below show the molecules covered in (a) to (k) above:
- Hydrogen molecule(H2) H–H
- Fluorine molecule(F2) F–F
- Chlorine molecule(Cl2) Cl–Cl
- Bromine molecule(Br2) Br–Br
- Iodine molecule(I2) I–I
- Oxygen molecule(O2) O=O
- Nitrogen molecule(N2) N=N
- Phosphorus molecule(P2) P=P
- Water molecule (H2O) H–O–H
j Ammonia molecule(NH3) H–N–H
H
k) Carbon (IV) oxide molecule (CO2) O==C==O
H
l) Methane molecule (CH4) H–C–H
H
Cl
m) Tetrachloromethane molecule (CCl4 Cl–C–Cl
Cl
H H
n) Ethane molecule (C2H6) H–C—C–H
H H
p) Ethene molecule (C2H4) H-C==C-H
H H
q) Ethyne molecule (C2H6) H-C—C-H
Dative /coordinate bond
A dative/coordinate bond is a covalent bond formed when a lone pair of electrons is donated then shared to an electron-deficient species/ion/atom.
During dative/coordinate bonding, all the shared pair of electrons are donated by one of the combining/bonding species/ ion/atom.
Like covalent bonding, coordinate /dative bond is mainly formed by non-metals.
Illustration of coordinate /dative bond
a) Ammonium ion (NH4+)
The ammonium ion is made up of ammonia (NH3) molecule and hydrogen (H+) ion. (H+) ion has no electrons. NH3 is made up of covalent bonding from Nitrogen and Hydrogen. One lone pair of electrons is present in Nitrogen atom after the bonding. This lone pair is donated and shared with an electron-deficient H+ ion
Diagram method 1
Diagram method 2
b) Phosphine ion (PH4+)
The Phosphine ion is made up of Phosphine (NH3) molecule and hydrogen (H+) ion. (H+) ion has no electrons. PH3 is made up of covalent bonding from Phosphorus and Hydrogen. One lone pair of electrons is present in Phosphorus atom. After the bonding this lone pair is donated and shared with the electron-deficient H+ ion
Diagram method 1
Diagram method 2
c) Hydroxonium (H3O+) ion
The hydroxonium ion is made up of water (H2O) molecule and hydrogen (H+) ion. (H+) ion has no electrons. The H2O molecule is made up of covalent bonding from Oxygen and Hydrogen. One lone pair of electrons out of the two present in Oxygen atom after the bonding is donated and shared with the electron-deficient H+ ion
Diagram method 1
Diagram method 2
d) Carbon (II) oxide (CO)
Carbon (II) oxide is made up of carbon and Oxygen atoms sharing each two outer electron and not sharing each two electrons. Oxygen with an extra lone pair of electrons donates and share with the carbon atom for both to be stable.
Diagram method 1
Diagram method 2
e) Aluminum (III) chloride (AlCl3/Al2Cl6)
Aluminum (III) chloride is made up of aluminum and chlorine. One aluminum atom shares its outer electrons with three separate chlorine atoms. All chlorine atoms attain stable octet but aluminum does not. Another molecule of aluminum chloride shares its chlorine lone pair of electrons with the aluminum atom for both to be stable. This type of bond exists only in vapour phase after aluminum chloride sublimes.
Diagram method 1
Diagram method 2
A dative/coordinate bond is by convention represented by an arrow (→) heading from the donor of the shared pair of electrons.
Below is the representation of molecules in the above examples;
a) Ammonium ion.
H
H− N→H
H
b) Phosphine ion H
H− P→H
H
c) Hydroxonium ion
H− O→H
H
d) Carbon (II) oxide O→C
d) Aluminum (III) chloride Cl Cl Cl
Al Al
Cl Cl Cl
(ii)IONIC/ELECTROVALENT BOND
An ionic/electrovalent bond is extreme of a covalent bond.
During ionic/electrovalent bonding there is complete transfer of valence electrons to one electronegative atom from an electropositive atom.
All metals are electropositive and easily/readily donate/lose their valence electrons.
All non-metals are electronegative and easily/readily gain/acquire extra electrons.
Ionic/electrovalent bonding therefore mainly involves transfer of electrons from metal/metallic radical to non-metallic radical.
When an electropositive atom donates /loses the valence electrons, it forms a positively charged cation to attain stable octet/duplet.
When an electronegative atom gains /acquires extra valence electrons, it forms a negatively charged anion to attain stable octet/duplet.
The electrostatic attraction force between the stable positively charged cation and the stable negatively charged anion with opposite charges constitute the ionic bond.
Like in covalent/dative/coordinate bonding, only the outer energy level electrons take part in the formation of ionic/electrovalent bond
Like in covalent/dative/coordinate bonding, the more electrons taking part / involved in the formation of ionic/electrovalent bond, the stronger the ionic /electrovalent bond. Illustration of ionic /electrovalent bond
a) Sodium chloride (NaCl)
Sodium chloride (NaCl) is formed when a sodium atom donates its outer valence electrons to chlorine atom for both to attain stable octet:
Symbol of atoms/elements taking part in bonding Na Cl
Number of protons/electrons 11 17
Electron configuration/structure 2:8:1 2:8:7
Number of electron in outer energy level 11 7
Number of electrons donated and gained to be stable 1 1
New electron configuration/structure 2:8: 2:8:
Symbol of cation/anion after bonding Na+ Cl–
Diagram
b) Magnesium chloride (MgCl2)
Magnesium chloride (MgCl2) is formed when a magnesium atom donate its two outer valence electrons to chlorine atoms. Two chlorine atoms are required to gain each one electron. All the ions (cations and anions) attain stable octet:
Symbol of atoms/elements taking part in bonding Mg Cl
Number of protons/electrons 11 17
Electron configuration/structure 2:8:2 2:8:7
Number of electron in outer energy level 2 7
Number of electrons donated and gained to be stable 2 1
New electron configuration/structure 2:8: 2:8:
Symbol of cation/anion after bonding Mg2+ Cl–
Diagram
c) Lithium oxide (Li2O)
Lithium oxide (Li2O) is formed when a Lithium atom donate its outer valence electrons to Oxygen atom. Two Lithium atoms are required to donate/lose each one electron and attain stable duplet. Oxygen atom acquires the two electrons and attain stable octet:
Symbol of atoms/elements taking part in bonding Li O
Number of protons/electrons 3 8
Electron configuration/structure 2:1 2:6
Number of electron in outer energy level 1 6
Number of electrons donated and gained to be stable 1 2
New electron configuration/structure 2: 2:8:
Symbol of cation/anion after bonding Li+ O2-
Diagram
d) Aluminum (III) oxide (Al2O3)
Aluminum (III) oxide (Al2O3) is formed when a Aluminum atom donate its three outer valence electrons to Oxygen atom. Two Aluminum atoms are required to donate/lose each three electron and attain stable octet. Three Oxygen atoms gain/ acquire the six electrons and attain stable octet:
Symbol of atoms/elements taking part in bonding Al O
Number of protons/electrons 13 8
Electron configuration/structure 2:8:3 2:6
Number of electron in outer energy level 3 6
Number of electrons donated and gained to be stable 3 2
New electron configuration/structure 2:8: 2:8:
Symbol of cation/anion after bonding Al3+ O2-
Diagram
e) Calcium oxide (CaO)
Calcium oxide (CaO) is formed when a Calcium atom donate its two outer valence electrons to Oxygen atom. Both attain stable octet:
Symbol of atoms/elements taking part in bonding Ca O
Number of protons/electrons 20 8
Electron configuration/structure 2:8:8:2 2:6
Number of electron in outer energy level 2 6
Number of electrons donated and gained to be stable 2 2
New electron configuration/structure 2:8:8: 2:8:
Symbol of cation/anion after bonding Ca2+ O2-
Diagram
Some compounds can be formed from ionic/electrovalent, covalent and dative/coordinate bonding within their atoms/molecules:
a) Formation of ammonium chloride:
Ammonium chloride is formed from the reaction of ammonia gas and hydrogen chloride gas. Both ammonia and hydrogen chloride gas are formed from covalent bonding. During the reaction of ammonia and hydrogen chloride gas to form Ammonium chloride;
-ammonia forms a dative/coordinate bond with electron deficient H+ ion from Hydrogen chloride to form ammonium ion(NH4+)ion.
-the chloride ion Cl– and ammonium ion(NH4+)ion bond through ionic / electrovalent bond from the electrostatic attraction between the opposite/unlike charges.
Diagram
b) Dissolution/dissolving of hydrogen chloride:
Hydrogen chloride is formed when hydrogen and chlorine atoms form a covalent bond. Water is formed when hydrogen and Oxygen atoms also form a covalent bond. When hydrogen chloride gas is dissolved in water;
-water molecules forms a dative/coordinate bond with electron deficient H+ ion from Hydrogen chloride to form hydroxonium ion(H3O+)ion.
-the chloride ion Cl– and hydroxonium ion(H3O+)ion bond through ionic / electrovalent bond from the electrostatic attraction between the opposite/unlike charges.
Diagram
.
c) Dissolution/dissolving of ammonia gas:
Ammonia gas is formed when hydrogen and Nitrogen atoms form a covalent bond. Water is formed when hydrogen and Oxygen atoms also form a covalent bond. When Ammonia gas is dissolved in water;
-ammonia forms a dative/coordinate bond with electron deficient H+ ion from a water molecule to form ammonium ion(NH4+)ion.
-the hydroxide ion OH– and ammonium ion(NH4+)ion bond through ionic / electrovalent bond from the electrostatic attraction between the opposite/unlike charges.
Diagram
(iii)METALLIC BOND
A metallic bond is formed when metallic atoms delocalize their outer electrons in order to be stable.
Metals delocalize their outer electrons to form positively charged cation .
The electrostatic attraction force between the metallic cation and the negatively charged electrons constitute the metallic bond.
The more delocalized electrons the stronger the metallic bond.
Illustration of ionic /electrovalent bond
a) Sodium (Na) is made of one valence electron. The electron is donated to form Na+ ion. The electron is delocalized /free within many sodium ions.
Symbol of atoms/elements taking part in bonding Na Na Na
Number of protons/electrons 11 11 11
Electron configuration/structure 2:8:1 2:8:1 2:8:1
Number of electron in outer energy level 1 1 1
Number of electrons delocalized/free within 1 1 1
New electron configuration/structure 2:8: 2:8: 2:8:
Symbol of cation after metallic bonding Na+ Na+ Na+
Diagram
(three)Metallic cations attract
(three) free/delocalized electrons
b) Aluminium (Al) is made of three valence electron. The three electrons are donated to form Al3+ ion. The electrons are delocalized /free within many aluminium ions.
Symbol of atoms/elements taking part in bonding Al Al Al
Number of protons/electrons 13 13 13
Electron configuration/structure 2:8:3 2:8:3 2:8:3
Number of electron in outer energy level 3 3 3
Number of electrons delocalized/free within 3 3 3
New electron configuration/structure 2:8: 2:8: 2:8:
Symbol of cation after metallic bonding Al3+ Al3+ Al3+
Diagram
(three)Metallic cations attract
(nine) free/delocalized electrons
c)Calcium (Ca) is made of two valence electron.The two electrons are donated to form Ca2+ ion.The electrons are delocalized /free within many Calcium ions.
Symbol of atoms/elements taking part in bonding Ca Ca Ca
Number of protons/electrons 20 20 20
Electron configuration/structure 2:8:8:2 2:8:8:2 2:8:8:2
Number of electron in outer energy level 2 2 2
Number of electrons delocalized/free within 2 2 2
New electron configuration/structure 2:8:8: 2:8:8: 2:8:8:
Symbol of cation after metallic bonding Ca2+ Ca2+ Ca2+
Diagram
(three)Metallic cations attract
(six) free/delocalized electrons
d) Magnesium (Mg) is made of two valence electron. The two electrons are donated to form Mg2+ion.The electrons are delocalized /free within many Magnesium ions.
Symbol of atoms/elements taking part in bonding Mg Mg
Number of protons/electrons 12 12
Electron configuration/structure 2:8:2 2:8:2
Number of electron in outer energy level 2 2
Number of electrons delocalized/free within 2 2
New electron configuration/structure 2:8: 2:8:
Symbol of cation after metallic bonding Mg2+ Mg2+
Diagram
(two)Metallic cations attract
(four) free/delocalized electrons
e)Lithium (Li) is made of one valence electron. The electron is donated to form Li+ ion. The electron is delocalized /free within many Lithium ions.ie;
Symbol of atoms/elements taking part in bonding Li Li Li Li
Number of protons/electrons 3 3 3 3
Electron configuration/structure 2:1 2:1 2:1 2:1
Number of electron in outer energy level 1 1 1 1
Number of electrons delocalized/free within 1 1 1 1
New electron configuration/structure 2:1: 2:1: 2:1: 2:1:
Symbol of cation after metallic bonding Li+ Li+ Li+ Li+
Diagram
(four)Metallic cations attract (four) free/delocalized electrons
