Chemical structure is the pattern/arrangement of atoms after they have bonded. There are two main types of chemical structures:
(i) Simple molecular structure
(ii) Giant structures
(i)Simple molecular structure
Simple molecular structure is the pattern formed after atoms of non-metals have covalently bonded to form simple molecules.
Molecules are made of atoms joined together by weak intermolecular forces called Van-der-waals forces.The Van-der-waals forces hold the molecules together while the covalent bonds hold the atoms in the molecule.
Illustration of simple molecular structure
a)Hydrogen molecule(H2)
Hydrogen gas is made up of strong covalent bonds/intramolecular forces between each hydrogen atom making the molecule. Each molecule is joined to another by weak Van-der-waals forces/ intermolecular forces.
Illustration of simple molecular structure
a)Hydrogen molecule(H2)
Hydrogen gas is made up of strong covalent bonds/intramolecular forces between each hydrogen atom making the molecule. Each molecule is joined to another by weak Van-der-waals forces/ intermolecular forces
b)Oxygen molecule(O2)
Oxygen gas is made up of strong covalent bonds/intramolecular forces between each Oxygen atom making the molecule. Each molecule is joined to another by weak Van-der-waals forces/ intermolecular forces.
Strong intramolecular forces/covalent bond
O=O:::: O=O:::: O=O:::: O=O
: : : : : : : : : : : : weak intermolecular
O=O:::: O=O:::: O=O:::: O=O forces/van-der-waals forces
c) Iodine molecule(I2)
Iodine solid crystals are made up of strong covalent bonds/intramolecular forces between each iodine atom making the molecule. Each molecule is joined to another by weak Van-der-waals forces/ intermolecular forces.
Strong intramolecular forces/covalent bond
I— I:::: I — I:::: I — I:::: I — I
: : : : : : : : : : : : : : weak intermolecular
I — I:::: I — I:::: I — I:::: I — I forces/van-der-waals forces
d)Carbon(IV) oxide molecule(CO2)
Carbon (IV) oxide gas molecule is made up of strong covalent bonds/intramolecular forces between each Carbon and oxygen atoms making the molecule. Each molecule is joined to another by weak Van-der-waals forces/ intermolecular forces.
Strong intramolecular forces/covalent bond
O=C=O:::: O=C=O:::: O=C=O
: : : : : : weak intermolecular
O=C=O:::: O=C=O:::: O=C=O forces/van-der-waals forces
The following are the main characteristic properties of simple molecular structured compounds:
a)State: Form Two Chemistry Notes
Most simple molecular substances are gases, liquid or liquids or solid that sublimes or has low boiling/melting points at room temperature (25oC) and pressure (atmospheric pressure).
Examples of simple molecular substances include:
-all gases eg Hydrogen, oxygen, nitrogen, carbon (IV) oxide,
–Petroleum fractions eg Petrol, paraffin, diesel, wax,
-Solid non-metals eg Sulphur, Iodine
-Water
b) Low melting/boiling points
Melting is the process of weakening the intermolecular/ van-der-waal forces/ of attraction between the molecules that holding the substance/compound.
Note;
(i)Melting and boiling does not involve weakening/breaking the strong intramolecular force/covalent bonds holding the atoms in the molecule.
(ii) Melting and boiling points increase with increase in atomic radius/size of the atoms making the molecule as the intermolecular forces / van-der-waal forces of attraction between the molecules increase. e.g.
Iodine has a higher melting/boiling point than chlorine because it has a higher /bigger atomic radius/size than chlorine, making the molecule to have stronger intermolecular force/ van-der-waal forces of attraction between the molecules than chlorine. Iodine is hence a solid and chlorine is a gas.
(c)Insoluble in water/soluble in organic solvents
Polar substances dissolve in polar solvents. Water is a polar solvent .Molecular substances do not thus dissolve in water because they are non-polar. They dissolve in non-polar solvents like methylbenzene, benzene, tetrachloromethane or propanone.
d) Poor conductors of heat and electricity
Substances with free mobile ions or free mobile/delocalized electrons conduct electricity. Molecular substances are poor conductors of heat/electricity because their molecules have no free mobile ions/electrons. This makes them very good insulators. Hydrogen bonds
A hydrogen bond is an intermolecular force of attraction in which a very electronegative atom attracts hydrogen atom of another molecule.
The most electronegative elements are Fluorine, Oxygen and Nitrogen .Molecular compounds made up of these elements usually have hydrogen bonds.
Hydrogen bonds are stronger than van-der-waals forces but weaker than covalent bonds. Molecular compounds with hydrogen bonds thus have higher melting/boiling points than those with van-der-waals forces.
Illustration of Hydrogen bonding
a)Water Molecule: Form Two Chemistry Notes
During formation of covalent bond, the oxygen atom attract/pull the shared electrons more to itself than Hydrogen creating partial negative charges(δ–)in Oxygen and partial positive charges(δ+)in Hydrogen. Two molecules attract each other at the partial charges through Hydrogen bonding
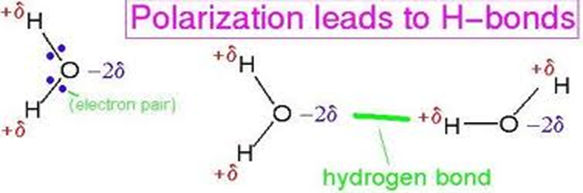
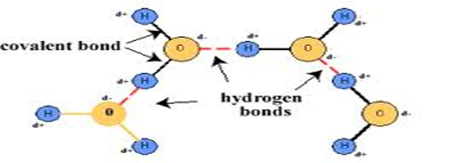
The hydrogen bonding in water makes it;
(i)a liquid with higher boiling and melting point than simple molecular substances with higher molecular mass. e.g. Hydrogen sulphide as in the table below;
Influence of H-bond in water (H2O) in comparison to H2S
| Substance | Water/ H2O | Hydrogen sulphide/ H2S |
| Relative molecular mass | 18 | 34 |
| Melting point(oC) | 0 | -85 |
| Boiling point(oC) | 100 | -60 |
(ii)have higher volume in solid (ice) than liquid (water) and thus ice is less dense than water. Ice therefore floats above liquid water.
b)Ethanol molecule
Like in water, the oxygen atom attracts/pulls the shared electrons in the covalent bond more to itself than Hydrogen.
This creates a partial negative charge (δ-) on oxygen and partial positive charge(δ+) on hydrogen.
Two ethanol molecules attract each other at the partial charges through Hydrogen bonding forming a dimmer.
A dimmer is a molecule formed when two molecules join together as below:
Hydrogen bonds covalent bonds
R1 O δ-…………………….…H δ+ O δ-
H δ+ R2
R1 and R2 are extensions of the molecule.
For ethanol it is made up of CH3CH2 – to make the structure:
Hydrogen bonds covalent bonds
CH3CH2 O δ-………………………….…H δ+ O δ-
H δ+ CH2CH3
b)Ethanoic acid molecule
Like in water and ethanol above, the oxygen atom attracts/pulls the shared electrons in the covalent bond in ethanoic acid more to itself than Hydrogen.
This creates a partial negative charge (δ-)on oxygen and partial positive charge(δ+) on hydrogen.
Two ethanoic acid molecules attract each other at the partial charges through Hydrogen-bonding forming a dimer.
Hydrogen bonds covalent bonds
R1 C O δ-………………………….…H δ+ O δ-
O δ- H δ+………………..….O δ- C R2
R1 and 2 are extensions of the molecule.
For ethanoic acid the extension is made up of CH3 – to make the structure;
Hydrogen bonds covalent bonds
CH3 C O δ-…………………………………….…H δ+ O δ-
O δ- H δ+…………………..……..………O δ- C CH3
Ethanoic acid like ethanol exists as a dimer.
Ethanoic acid has a higher melting/boiling point than ethanol .This is because ethanoic acid has two/more hydrogen bond than ethanol.
d) Proteins and sugars in living things also have multiple/complex hydrogen bonds in their structures.
(ii) Giant structure
This is the pattern formed after substances /atoms /ions bond to form a long chain network.
Giant structures therefore extend in all directions to form a pattern that continues repeating itself.
There are three main giant structures.
a) giant covalent/atomic structure b)giant ionic structure
c)giant metallic structure
a) giant covalent/atomic structure
Giant covalent/atomic structure is the pattern formed after atoms have covalently bonded to form long chain pattern consisting of indefinite number of atoms covalently bonded together.
The strong covalent bonds hold all the atoms together to form a very well packed structure. Examples of substances with giant covalent/atomic structure include:
(i) carbon-diamond
(ii) carbon-graphite
(iii)silicon
(iv) silicon(IV) oxide/sand
Carbon-graphite and carbon-diamond are allotropes of carbon.
Allotropy is the existence of an element in more than one stable physical form at the same temperature and pressure.
Allotropes are atoms of the same element existing in more than one stable physical form at the same temperature and pressure.
Other elements that exhibit/show allotropy include;
-Sulphur as monoclinic sulphur and rhombic sulphur
-Phosphorus as white phosphorus and red phosphorus
The Structure of Carbon-Diamond: Form Two Chemistry Notes
Carbon has four valence electrons. The four valence electrons are used to form covalent bonds. During the formation of diamond, one carbon atom covalently bond with four other carbon atoms.
C C
x x.
x C x —–> C .x C x. C ——> C C C
x x.
C C
After the bonding, the atoms rearrange to form a regular tetrahedral in which one carbon is in the centre while four are at the apex/corners.
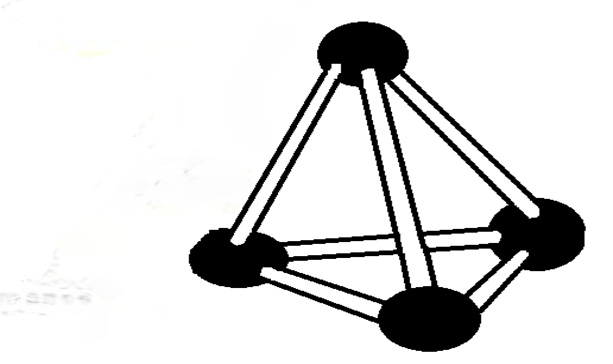
This pattern repeats itself to form a long chain number of atoms covalently bonded together indefinitely. The pattern is therefore called giant tetrahedral structure. It extends in all directions where one atom of carbon is always a centre of four others at the apex/corner of a regular tetrahedral.
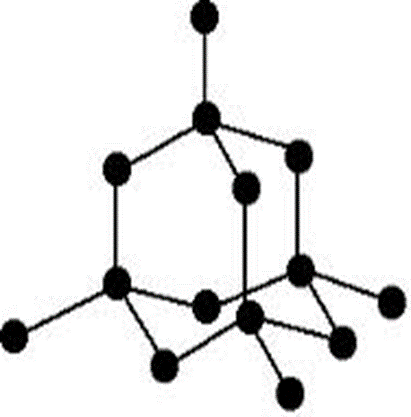
The giant tetrahedral structure of carbon-diamond is very well/closely packed and joined/bonded together by strong covalent bond.
This makes carbon-diamond to have the following properties:
a) High melting/boiling point.
The giant tetrahedral structure is very well packed and joined together by strong covalent bonds.
This requires a lot of energy/heat to weaken for the element to melt and break for the element to boil.
b) High density.
Carbon diamond is the hardest known natural substance.
This is because the giant tetrahedral structure is a very well packed pattern/structure and joined together by strong covalent bonds.
This makes Carbon diamond be used to make drill for drilling boreholes/oil wells.
The giant tetrahedral structure of carbon diamond is a very closely packed pattern /structure such that heat transfer by conduction is possible. This makes carbon diamond a good thermal conductor.
c) Poor conductor of electricity.
Carbon-diamond has no free/delocalized electrons within its structure and thus do not conduct electricity.
d) Insoluble in water.
Carbon-diamond is insoluble in water because it is non-polar and do not bond with water molecules.
e) Is abrasive/Rough.
The edges of the closely well packed pattern/structure of Carbon-diamond make its surface rough/abrasive and thus able to smoothen /cut metals and glass.
f) Have characteristic luster.
Carbon-diamond has a high optical dispersion and thus able to disperse light to different colours .This makes Carbon-diamond one of the most popular gemstone for making jewellery.
The structure of carbon-graphite
During the formation of graphite, one carbon atom covalently bond with three other carbon atoms leaving one free/delocalized electron.
C C
x x.
x C x —–> C .x C x ——> C C x free/delocalized electron
x x.
C C
After the bonding, the atoms rearrange and join together to form a regular hexagon in which six carbon atoms are at the apex/corners.
The regular hexagon is joined to another in layers on the same surface by van-der-waals forces.
Each layer extends to form a plane in all directions.
The fourth valence electron that does not form covalent bonding is free/mobile /delocalized within the layers.
This structure/pattern is called giant hexagonal planar structure.
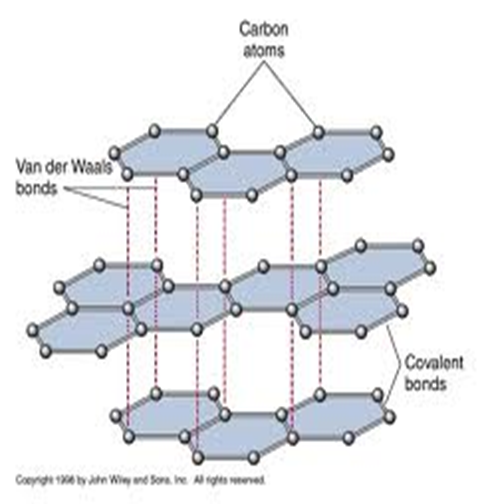
The giant hexagonal planar structure of carbon-graphite is closely packed and joined/bonded together by strong covalent bonds. This makes carbon-graphite to have the following properties:
a) High melting/boiling point.
The giant hexagonal planar structure of carbon-graphite is well packed and joined together by strong covalent bonds.
This requires a lot of energy/heat to weaken for the element to melt and break for the element to boil.
b) Good conductor of electricity.
Carbon-graphite has free/delocalized 4th valence electrons within its structure and thus conducts electricity.
c) Insoluble in water.
Carbon-graphite is insoluble in water because it is non-polar and do not bond with water molecules.
d) Soft.
Layers of giant hexagonal planar structure of carbon graphite are held together by van-der-waals forces.
The van-der-waals forces easily break when pressed and reform back on releasing/reducing pressure/force thus making graphite soft.
e) Smooth and slippery.
When pressed at an angle the van-der-waals forces easily break and slide over each other making graphite soft and slippery.
It is thus used as a dry lubricant instead of oil.
f)Uses of Carbon-Graphite.: Form Two Chemistry Notes
1. As a dry lubricant- carbon graphite is smooth and slippery and thus better lubricant than oil.Oil heat up when reducing friction.
2. Making Lead-pencils- When pressed at an angle on paper the van-der-waals forces easily break and slide smoothly over contrasting background producing its characteristic black background.
3. As moderator in nuclear reactors to reduce the rate of decay/disintegration of radioactive nuclides/atoms/isotopes.
4. As electrode in dry/wet cells/battery- carbon graphite is inert and good conductor of electricity. Current is thus able to move from one electrode/terminal to the other in dry and wet cells/batteries. Carbon graphite is also very cheap.
b) giant ionic structure
Giant ionic structure is the pattern formed after ions have bonded through ionic/electrovalent bonding to form a long chain consisting of indefinite number of ions.
The strong ionic/electrovalent bond holds all the cations and anions together to form a very well packed structure.
Substances with giant ionic structure are mainly crystals of salts e.g. sodium chloride, Magnesium chloride, Sodium iodide, Potassium chloride, copper (II) sulphate(VI).
The structure of sodium chloride
Sodium chloride is made up of sodium (Na+) and chloride (Cl–)ions.
Sodium (Na+) ion is formed when a sodium atom donate /loose/donate an electron. Chloride (Cl–) ion is formed when a chlorine atom gain /acquire an extra electron from sodium atom.
Many Na+ and Cl– ions then rearrange such that one Na+ ion is surrounded by six Cl– ions and one Cl– ion is surrounded by six Na+ ions.
The pattern formed is a giant cubic structure where Cl– ion is sand witched between Na+ ions and the same to Na+ ions.
This pattern forms a crystal.
A crystal is a solid form of a substance in which particles are arranged in a definite pattern regularly repeated in three dimensions.
The Structure of Sodium Chloride: Form Two Chemistry Notes
The giant cubic structure/crystal of sodium chloride is as below;
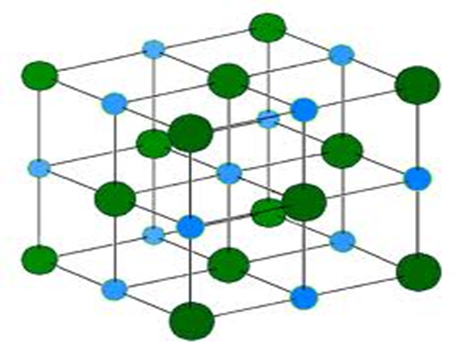
The giant cubic structure/crystal of sodium chloride is very well packed and joined by strong ionic/electrovalent bonds. This makes sodium chloride and many ionic compounds to have the following properties:
a) Have high melting /boiling points.
The giant cubic lattice structure of sodium chloride is very closely packed into a crystal that requires a lot of energy/heat to weaken and melt/boil. This applies to all crystalline ionic compounds.
b) Are good conductors of electricity in molten and aqueous state but poor conductor of electricity in solid.
Ionic compounds have fused ions in solid crystalline state.
On heating and dissolving in water, the crystal is broken into free mobile ions (Na+ and Cl– ions).
The free mobile ions are responsible for conducting electricity in ionic compounds in molten and aqueous states.
c)Soluble in water
Ionic compounds are polar and dissolve in polar water molecules.
On dissolving, the crystal breaks to free the fused ions which are then surrounded by water molecules.
b) giant metallic structure
This is the pattern formed after metallic atoms have bonded through metallic bond.
The pattern formed is one where the metallic cations rearrange to form a cubic structure.
The cubic structure is bound together by the free delocalized electrons that move freely within.
The more delocalized electrons, the stronger the metallic bond.
The structure of sodium and aluminium.
Sodium has one valence electrons.
Aluminium has three valence electrons.
After delocalizing the valence electrons ,the metal cations (Na+ and Al3+) rearrange to the apex /corners of a regular cube that extend in all directions.
The delocalized electrons remain free and mobile as shown below:
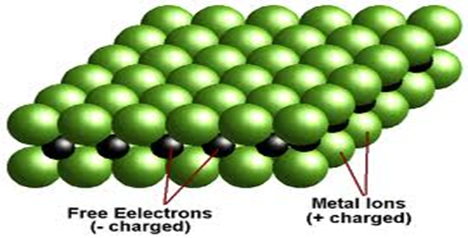
The giant cubic structure makes metals to have the following properties:
a) Have high melting/boiling point
The giant cubic structure is very well packed and joined/bonded together by the free delocalized electrons.
The more delocalized electrons the higher the melting/boiling point.
The larger/bigger the metallic cation, the weaker the packing of the cations and thus the lower the melting/boiling point. e.g.
(i) Sodium and potassium have both one valence delocalized electron.
Atomic radius of potassium is larger/bigger than that of sodium and hence less well packed in its metallic structure.
Sodium has therefore a higher melting/boiling point than potassium.
(ii) Sodium has one delocalized electron.
Aluminium has three delocalized electrons.
Atomic radius of sodium is larger/bigger than that of aluminium and hence less well packed in its metallic structure.
Aluminium has therefore a higher melting/boiling point than sodium because of the smaller well packed metallic (Al3+) ions and bonded/joined by more/three delocalized electrons.
The table below shows the comparative melting/boiling points of some metals:
| Metal | Electronic structure | Atomic radius(nM) | Melting point(oC) | Boiling point(oC) |
| Sodium | 2:8:1 | 0.155 | 98 | 890 |
| Potassium | 2:8:8:1 | 0.203 | 64 | 774 |
| Magnesium | 2:8:2 | 0.136 | 651 | 1110 |
| Aluminium | 2:8:3 | 0.125 | 1083 | 2382 |
b) Good electrical and thermal conductor/electricity.
All metals are good conductors of heat and electricity including Mercury which is a liquid.
The mobile delocalized electrons are free within the giant metallic structure to move from one end to the other transmitting heat/electric current.
The more delocalized electrons the better the thermal/electrical conductivity.
High temperatures/heating lowers the thermal/electrical conductivity of metals because the delocalized electrons vibrate and move randomly hindering transfer of heat
From the table above:
Compare the electrical conductivity of:
(i)Magnesium and sodium
Magnesium is a better conductor than sodium.
Magnesium has more/two delocalized electrons than sodium. The more delocalized electrons the better the electrical conductor.
(ii)Potassium and sodium
Potassium is a better conductor than sodium.
Potassium has bigger/larger atomic radius than sodium. The delocalized electrons are less attracted to the nucleus of the atom and thus more free /mobile and thus better the electrical conductor.
c) Insoluble in water
All metals are insoluble in water because they are non polar and thus do not bond with water.
Metals higher in the reactivity/electrochemical series like; Potassium, sodium, Lithium and Calcium reacts with cold water producing hydrogen gas and forming an alkaline solution of their hydroxides.ie
2K(s) + 2H2O(l) -> 2KOH(aq) + H2(g)
2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2(g)
2Li(s) + 2H2O(l) -> 2LiOH(aq) + H2(g)
Ca(s) + 2H2O(l) -> Ca(OH)2(aq)+ H2(g)
Heavy metal like Magnesium, Aluminium, Iron, Zinc and Lead react with steam/water vapour to produce hydrogen gas and form the corresponding oxide.
Mg(s) + H2O(g) -> MgO(s) + H2(g)
Fe(s) + H2O(g) -> FeO(s) + H2(g)
Zn(s) + H2O(g) -> ZnO(s) + H2(g)
Pb(s) + H2O(g) -> PbO(s) + H2(g)
2Al(s) + 3H2O(g) -> Al2O3(s) + 3H2(g)
Metals lower in the reactivity/electrochemical series than hydrogen like; copper, Mercury, Gold Silver and Platinum do not react with water/vapour.
d) Shiny metallic-lustre
All metals have a shiny grey metallic luster except copper which is brown.
When exposed to sunlight, the delocalized electrons gain energy, they vibrate on the metal surface scattering light to appear shiny.
With time, most metals corrode and are covered by a layer of the metal oxide.
The delocalized electrons are unable to gain and scatter light and the metal surface tarnishes/become dull.
e) Ductile and malleable
All metals are malleable (can be made into thin sheet) and ductile (can be made into wire.
When beaten/hit/pressed lengthwise the metallic cations extend and is bound /bonded by the free/mobile electrons to form a sheet.
When beaten/hit/pressed lengthwise and bredthwise the metallic cations extend and is bound /bonded by the free/mobile electrons to form a wire/thin strip.
f) Have high tensile strength
Metals are not brittle. The free delocalized electrons bind the metal together when it is bent /coiled at any angle.
The metal thus withstand stress/coiling
g) Form alloys
An alloy is a uniform mixture of two or more metals.
Some metals have spaces between their metallic cations which can be occupied by another metal cation with smaller atomic radius.
Common alloys include:
Brass(Zinc and Copper alloy)
Bronze(Copper and Tin alloy)
German silver
Summary of Bonding and Structure: Form Two Chemistry Notes
| Simple molecular structure | Giant covalent /atomic structure | Giant ionic structure | Giant metallic structure | |
| (i)Examples | I2,S8,HCl,O2,CH4 | Graphite,diamond Si,SiO2 | NaCl, KCl, CaO,CuSO4 | Na,Fe,Cr,Hg,K |
| Constituent particles making structure | molecules | Atoms (of non-metals) | Ions (cation and anions) | Atoms (of metals) |
| Type of substance | Non-metal element/non-metal molecule/non-metal compound(electronegative elements) | Group IV non-metals and some of their oxides | Metal-non metal compounds(compounds of electropositive and electronegative compounds) | Metallic compounds Metallic elements (with low electonegativity and high electropositivity) |
| Bonding in solid state | -Strong covalent bonds hold atoms together within separate molecules (intramolecular forces) -Weak van-der-waals forces hold separate molecules together (intermolecular forces) | Atoms are linked through the whole structure by very strong covalent bonds. | Electrostatic attraction of cations and anions link the whole structure through strong ionic bond. | EEElectrostatic Electrostatic attraction of outer mobile electrons for positive nuclei binds atoms together though metallic bond |
| Properties (i) Volatility | -Highly volatile with low melting/boiling point -Low latent heat of fusion/vaporization | -Non volatile with very high melting/boiling points -Low latent heat of fusion / vaporization | -Non volatile with very high melting/boiling points -Low latent heat of fusion / vaporization | -Non volatile with very high melting/boiling points -Low latent heat of fusion / vaporization |
| (ii) State at room temperature /pressure | Usually gases,volatile liquids or solids that sublimes | solids | solids | Solids except Mercury(liquid) |
| (iii) Hardness | Soft and brittle(low tensile strength) | Hard and brittle(low tensile strength) | Hard and brittle(low tensile strength) | Hard, malleable, ductile and have high tensile strength |
| (iv) Thermal /electrical conductivity | Poor thermal and electrical conductor when solid ,liquid or aqueous solutions but some dissolve and react to form electrolytes e.g. Hydrogen chloride and ammonia gases. | Poor thermal and electrical conductor when solid ,liquid or aqueous solutions but -Carbon-graphite is a good electrical conductor while -Carbon-diamond is a good thermal conductor. | Poor thermal and electrical conductor when solid. Good thermal and electrical conductor in liquid/molten and aqueous states when the ions are not fused | Good thermal and electrical conductor in solid and liquid/molten states due to the free mobile /delocalized electrons |
| (v) Solubility | Insoluble in polar solvents e.g. Water Soluble in non-polar solvents e.g. tetrachloromethane, benzene, methylbenzene | Insoluble in all solvents | Soluble in polar solvents e.g. Water Insoluble in non-polar solvents e.g. tetrachloromethane, benzene, methylbenzene | Insoluble in polar/non-polar colvents. -Some react with polar solvents -Some metal dissolve in other metals to form alloys e.g. Brass is formed when Zinc dissolve in copper. |
