1. A fixed mass of gas at 102300Pa pressure has a volume of 25cm3.Calculate its volume if the pressure is doubled.
Working
P1 V1 = P2 V2 Substituting :102300 x 25 = (102300 x 2) x V2
V2 = 102300 x 25 = 12.5cm3
(102300 x 2)
2. Calculate the pressure which must be applied to a fixed mass of 100cm3 of Oxygen for its volume to triple at 100000Nm-2.
P1 V1 = P2 V2 Substituting :100000 x 100 = P2 x (100 x 3)
V2 = 100000 x 100 = 33333.3333 Nm-2
(100 x 3)
3.A 60cm3 weather ballon full of Hydrogen at atmospheric pressure of 101325Pa was released into the atmosphere. Will the ballon reach stratosphere where the pressure is 90000Pa?
P1 V1 = P2 V2 Substituting :101325 x 60 = 90000 x V2
V2 = 101325 x 60 = 67.55 cm3
90000
The new volume at 67.55 cm3 exceed ballon capacity of 60.00 cm3.It will burst before reaching destination.
7.Charles law states that“the volume of a fixed mass of a gas is directly proportional to the absolute temperature at constant/fixed pressure ”
Mathematically:
Volume α Pressure (Fixed /constant pressure)
V α T (Fixed /constant P) ie V = Constant(k)
T
From Charles law , an increase in temperature of a gas cause an increase in volume. i.e doubling the temperature cause the volume to be doubled.
Gases expand/increase by 1/273 by volume on heating.Gases contact/decrease by 1/273 by volume on cooling at constant/fixed pressure.
The volume of a gas continue decreasing with decrease in temperature until at -273oC /0 K the volume is zero. i.e. there is no gas.
This temperature is called absolute zero. It is the lowest temperature at which a gas can exist.
Graphically therefore a plot of volume(V) against Temperature(T) in:
(i)oC produces a straight line that is extrapolated to the absolute zero of -273oC .
(ii)Kelvin/K produces a straight line from absolute zero of O Kelvin
4. 500cm3 of carbon(IV)oxide at 0oC was transfered into a cylinder at -4oC. If the capacity of the cylinder is 450 cm3,explain what happened.
V1 = V2 substituting 500 = V2
T1 T2 (0 +273) (-4 +273)
= 500 x (-4 x 273) = 492.674cm3
(0 + 273)
The capacity of cylinder (500cm3) is less than new volume(492.674cm3).
7.326cm3(500-492.674cm3)of carbon(IV)oxide gas did not fit into the cylinder.
5. A mechanic was filling a deflated tyre with air in his closed garage using a hand pump. The capacity of the tyre was 40,000cm3 at room temperature. He rolled the tyre into the car outside. The temperature outside was 30oC.Explain what happens.
V1 = V2 substituting 40000 = V2
T1 T2 (25 +273) (30 +273)
= 40000 x (30 x 273) = 40671.1409cm3
(25 + 273)
The capacity of a tyre (40000cm3) is less than new volume(40671.1409cm3).
The tyre thus bursts.
6. A hydrogen gas balloon with 80cm3 was released from a research station at room temperature. If the temperature of the highest point it rose is -30oC , explain what happened.
V1 = V2 substituting 80 = V2
T1 T2 (25 +273) (-30 +273)
= 80 x (-30 x 273) = 65.2349cm3
(25 + 273)
The capacity of balloon (80cm3) is more than new volume (65.2349cm3).
The balloon thus remained intact.
7. The continuous random motion of gases differ from gas to the other.The movement of molecules (of a gas) from region of high concentration to a region of low concentration is called diffusion.
The rate of diffusion of a gas depends on its density. i.e. The higher the rate of diffusion, the less dense the gas.
The density of a gas depends on its molar mass/relative molecular mass. i.e. The higher the density the higher the molar mass/relative atomic mass and thus the lower the rate of diffusion.
Examples
1.Carbon (IV)oxide(CO2) has a molar mass of 44g.Nitrogen(N2)has a molar mass of 28g. (N2)is thus lighter/less dense than Carbon (IV)oxide(CO2). N2 diffuses faster than CO2.
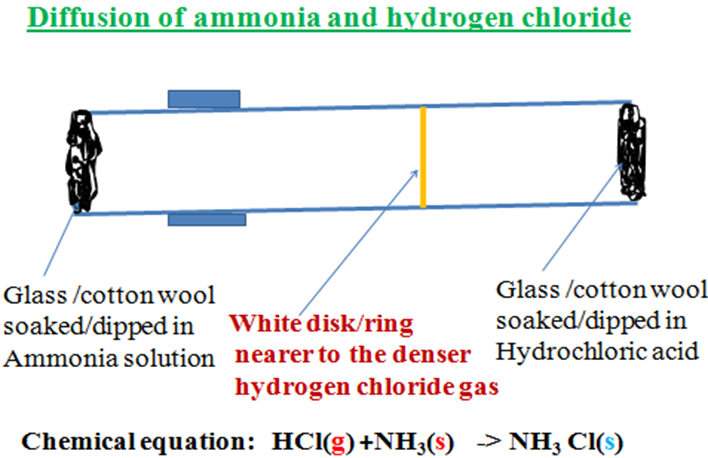
2.Ammonia(NH3) has a molar mass of 17g.Nitrogen(N2)has a molar mass of 28g. (N2)is thus about twice lighter/less dense than Ammonia(NH3). Ammonia(NH3)diffuses twice faster than N2. 3. Ammonia(NH3) has a molar mass of 17g.Hydrogen chloride gas has a molar mass of 36.5g.Both gases on contact react to form white fumes of ammonium chloride .When a glass/cotton wool dipped in ammonia and another glass/cotton wool dipped in hydrochloric acid are placed at opposite ends of a glass tube, both gases diffuse towards each other. A white disk appears near to glass/cotton wool dipped in hydrochloric acid. This is because hydrogen chloride is heavier/denser than Ammonia and thus its rate of diffusion is lower
The rate of diffusion of a gas is in accordance to Grahams law of diffusion.Grahams law states that:
“the rate of diffusion of a gas is inversely proportional to the square root of its density, at the same/constant/fixed temperature and pressure”
Mathematically
R α 1 and since density is proportional to mass then R α 1
√ p √ m
For two gases then:
R1 = R2 where: R1 and R2 is the rate of diffusion of 1st and 2nd gas.
√M2 √M1 M1 and M2 is the molar mass of 1st and 2nd gas.
Since rate is inverse of time. i.e. the higher the rate the less the time:
For two gases then:
T1 = T2 where: T1 and T2 is the time taken for 1st and 2nd gas to diffuse.
√M1 √M2 M1 and M2 is the molar mass of 1st and 2nd gas.
8.
It takes 30 seconds for 100cm3 of carbon(IV)oxide to diffuse across a porous plate. How long will it take 150cm3 of nitrogen(IV)oxide to diffuse across the same plate under the same conditions of temperature and pressure. (C=12.0,N=14.0=16.0)
Molar mass CO2=44.0 Molar mass NO2=46.0
Method 1
100cm3 CO2 takes 30seconds
150cm3 takes 150 x30 = 45seconds
100
T CO2 = √ molar mass CO2 => 45seconds = √ 44.0
T NO2 √ molar mass NO2 T NO2 √ 46.0
T NO2 =45seconds x √ 46.0 = 46.0114 seconds
√ 44.0
Method 2
100cm3 CO2 takes 30seconds
1cm3 takes 100 x1 = 3.3333cm3sec-1
30
R CO2 = √ molar mass NO2 => 3.3333cm3sec-1 = √ 46.0
R NO2 √ molar mass CO2 R NO2 √ 44.0
R NO2 = 3.3333cm3sec-1 x √ 44.0 = 3.2601cm3sec-1
√ 46.0
3.2601cm3 takes 1seconds
150cm3 take 150cm3 = 46.0109seconds
3.2601cm3
9. How long would 200cm3 of Hydrogen chloride take to diffuse through a porous plug if carbon(IV)oxide takes 200seconds to diffuse through.
Molar mass CO2 = 44g Molar mass HCl = 36.5g
T CO2 = √ molar mass CO2 => 200 seconds = √ 44.0
T HCl √ molar mass HCl T HCl √ 36.5
T HCl = 200seconds x √ 36.5 = 182.1588 seconds
√ 44.0
10. Oxygen gas takes 250 seconds to diffuse through a porous diaphragm. Calculate the molar mass of gas Z which takes 227 second to diffuse.
Molar mass O2 = 32g Molar mass Z = x g
T O2 = √ molar mass O2 => 250 seconds = √ 32.0
T Z √ molar mass Z 227seconds √ x
√ x = 227seconds x √ 32 = 26.3828 grams
250
11. 25cm3 of carbon(II)oxide diffuses across a porous plate in 25seconds. How long will it take 75cm3 of Carbon(IV)oxide to diffuse across the same plate under the same conditions of temperature and pressure. (C=12.0,0=16.0)
Molar mass CO2 = 44.0 Molar mass CO = 28.0
Method 1
25cm3 CO takes 25seconds
75cm3 takes 75 x25 = 75seconds
25
T CO2 = √ molar mass CO2 => T CO2seconds = √ 44.0
T CO √ molar mass CO 75 √ 28.0
T CO2 =75seconds x √ 44.0 = 94.0175 seconds
√ 28.0
Method 2
25cm3 CO2 takes 25seconds
1cm3 takes 25 x1 = 1.0cm3sec-1
25
R CO2 = √ molar mass CO => x cm3sec-1 = √ 28.0
R CO √ molar mass CO2 1.0cm3sec-1 √ 44.0
R CO2 = 1.0cm3sec-1 x √ 28.0 = 0.7977cm3sec-1
√ 44.0
0.7977cm3 takes 1 seconds
75cm3 takes 75cm3 = 94.0203seconds
0.7977cm3
