1. Calculate the volume of Oxygen required to completely react with 50cm3 of Hydrogen.
Chemical equation: 2H2 (g) + O2 (g) -> 2H2O(l)
Volume ratios 2 : 1 : 0
Reacting volumes 50cm3 : 25cm3
50cm3 of Oxygen is used
2. Calculate the volume of air required to completely reacts with 50cm3 of Hydrogen.(assume Oxygen is 21% by volume of air)
Chemical equation: 2H2 (g) + O2 (g) -> 2H2O(l)
Volume ratios 2 : 1 : 0
Reacting volumes 50cm3 : 25cm3
50cm3 of Oxygen is used
21% = 25cm3
100% = 100 x 25 =
21
3.If 5cm3 of a hydrocarbon CxHy burn in 15cm3 of Oxygen to form 10cm3 of Carbon(IV)oxide and 10cm3 of water vapour/steam, obtain the equation for the reaction and hence find the value of x and y in CxHy.
Chemical equation: CxHy (g) + O2 (g) -> H2O(g) + CO2(g)
Volumes 5cm3 : 15cm3 : 10cm3 : 10cm3
Volume ratios 5cm3 : 15cm3 : 10cm3 : 10cm3 (divide by lowest volume) 5 5 5 5
Reacting volume ratios 1volume 3 volume 2 volume 2 volume
Balanced chemical equation: CxHy (g) + 3O2 (g) -> 2H2O(g) + 2CO2(g)
If “4H” are in 2H2O(g) the y=4
If “2C” are in 2CO2 (g) the x=2
Thus(i) chemical formula of hydrocarbon = C2H4
(ii) chemical name of hydrocarbon = Ethene
4.100cm3 of nitrogen (II)oxide NO combine with 50cm3 of Oxygen to form 100cm3 of a single gaseous compound of nitrogen. All volumes measured at the same temperature and pressure. Obtain the equation for the reaction and name the gaseous product.
Chemical equation: NO (g) + O2 (g) -> NOx
Volumes 100cm3 : 50cm3 : 100
Volume ratios 100cm3 : 50cm3 : 100cm3 (divide by lowest volume) 50 50 50
Reacting volume ratios 2volume 1 volume 2 volume
Balanced chemical equation: 2 NO (g) + O2 (g) -> 2NO x(g)
Thus(i) chemical formula of the nitrogen compound = 2 NO2
(ii) chemical name of compound = Nitrogen(IV)oxide
5.When 15cm3 of a gaseous hydrocarbon was burnt in 100cm3 of Oxygen ,the resulting gaseous mixture occupied70cm3 at room temperature and pressure. When the gaseous mixture was passed through, potassium hydroxide its volume decreased to 25cm3.
(a)What volume of Oxygen was used during the reaction.(1mk)
Volume of Oxygen used =100-25 =75cm3√
(P was completely burnt)
(b)Determine the molecular formula of the hydrocarbon(2mk)
CxHy + O2 -> xCO2 + yH2O
15cm3 : 75cm3
15 15
1 : 3√
=> 1 atom of C react with 6 (3×2)atoms of Oxygen
Thus x = 1 and y = 2 => P has molecula formula CH4√
(g) Ionic equations
An ionic equation is a chemical statement showing the movement of ions (cations and anions ) from reactants to products.
Solids, gases and liquids do not ionize/dissociate into free ions. Only ionic compounds in aqueous/solution or molten state ionize/dissociate into free cations and anions (ions)
An ionic equation is usually derived from a stoichiometric equation by using the following guidelines
Guidelines for writing ionic equations
1.Write the balanced stoichiometric equation
2.Indicate the state symbols of the reactants and products
3.Split into cations and anions all the reactants and products that exist in aqueous state.
4.Cancel out any cation and anion that appear on both the product and reactant side.
5. Rewrite the chemical equation. It is an ionic equation.
Practice
(a)Precipitation of an insoluble salt
All insoluble salts are prepared in the laboratory from double decomposition /precipitation. This involves mixing two soluble salts to form one soluble and one insoluble salt
1. When silver nitrate(V) solution is added to sodium chloride solution,sodium nitrate(V) solution and a white precipitate of silver chloride are formed.
Balanced stoichiometric equation
AgNO3(aq) + NaCl(aq) -> AgCl (s) + NaNO3 (aq)
Split reactants product existing in aqueous state as cation/anion
Ag+(aq) + NO3– (aq) + Na+(aq) + Cl–(aq) -> AgCl(s) + Na+(aq)+ NO3– (aq)
Cancel out ions appearing on reactant and product side
Ag+(aq) + NO3– (aq) + Na+(aq) + Cl–(aq) -> AgCl(s) + Na+(aq)+ NO3– (aq)
Rewrite the equation
Ag+(aq) + Cl–(aq) -> AgCl(s) (ionic equation)
2. When barium nitrate(V) solution is added to copper(II)sulphate(VI) solution, copper(II) nitrate(V) solution and a white precipitate of barium sulphate(VI) are formed.
Balanced stoichiometric equation
Ba(NO3)2(aq) + CuSO4(aq) -> BaSO4 (s) + Cu(NO3) 2 (aq)
Split reactants product existing in aqueous state as cation/anion
Ba2+(aq) + 2NO3– (aq) + Cu2+(aq) + SO42-(aq) -> BaSO4 (s) + 2NO3– (aq)+ Cu2+(aq)
Cancel out ions appearing on reactant and product side
Ba2+(aq) + 2NO3– (aq) +Cu2+ (aq) + SO42-(aq)-> BaSO4(s) + 2NO3– (aq) + Cu2+(aq)
Rewrite the equation
Ba2+(aq) + SO42-(aq) -> BaSO4(s) (ionic equation)
3.A yellow precipitate of Potassium Iodide is formed from the reaction of Lead(II)nitrate(v) and potassium iodide.
Balanced stoichiometric equation
Pb(NO3)2(aq) + 2KI(aq) -> PbI2 (s) + 2KNO3 (aq)
Split reactants product existing in aqueous state as cation/anion
Pb2+(aq) + 2NO3– (aq) + 2K +(aq) + 2I – (aq) -> PbI2 (s) + 2NO3– (aq)+ 2K +(aq)
Cancel out ions appearing on reactant and product side
Pb2+(aq) + 2NO3– (aq) + 2K +(aq) + 2I – (aq) -> PbI2 (s) + 2NO3– (aq)+ 2K +(aq)
Rewrite the equation
Pb2+(aq) + 2I– (aq) -> PbI2 (s) (ionic equation)
(b)Neutralization
Neutralization is the reaction of an acid with a soluble base/alkali or insoluble base.
(i)Reaction of alkalis with acids
1.Reaction of nitric(V)acid with potassium hydroxide
Balanced stoichiometric equation
HNO3(aq) + KOH(aq) -> H2O (l) + KNO3 (aq)
Split reactants product existing in aqueous state as cation/anion
H+(aq) + NO3– (aq) + K +(aq) + OH – (aq) -> H2O (l) + NO3– (aq)+ K +(aq)
Cancel out ions appearing on reactant and product side
H+(aq) + NO3– (aq) + K +(aq) + OH – (aq) -> H2O (l) + NO3– (aq)+ K +(aq)
Rewrite the equation
H+ (aq) + OH – (aq) -> H2O (l) (ionic equation)
2.Reaction of sulphuric(VI)acid with ammonia solution
Balanced stoichiometric equation
H2SO4(aq) + 2NH4OH(aq) -> H2O (l) + (NH4) 2SO4 (aq)
Split reactants product existing in aqueous state as cation/anion
2H+(aq) + SO42- (aq) + 2NH4 +(aq)+ 2OH – (aq) ->2H2O (l) +SO42- (aq)+ 2NH4 + (aq)
Cancel out ions appearing on reactant and product side
2H+(aq) + SO42- (aq) + 2NH4 +(aq)+ 2OH – (aq) ->2H2O (l) +SO42- (aq)+ 2NH4 + (aq)
Rewrite the equation
2H+ (aq) + 2OH – (aq) -> 2H2O (l)
H+ (aq) + OH – (aq) -> H2O (l) (ionic equation)
3.Reaction of hydrochloric acid with Zinc hydroxide
Balanced stoichiometric equation
2HCl(aq) + Zn(OH)2 (s) -> 2H2O (l) + ZnCl 2 (aq)
Split reactants product existing in aqueous state as cation/anion
2H+(aq) + 2Cl– (aq) + Zn(OH)2 (s) ->2H2O (l) + 2Cl– (aq)+ Zn 2+ (aq)
Cancel out ions appearing on reactant and product side
2H+(aq) + 2Cl– (aq) + Zn(OH)2 (s) ->2H2O (l) + 2Cl– (aq)+ Zn 2+ (aq)
Rewrite the equation
2H+(aq) + Zn(OH)2 (s) ->2H2O (l) + Zn 2+ (aq) (ionic equation)
(h)Molar solutions
A molar solution is one whose concentration is known. The SI unit of concentration is Molarity denoted M.
Molarity may be defined as the number of moles of solute present in one cubic decimeter of solution.
One cubic decimeter is equal to one litre and also equal to 1000cm3.
The higher the molarity the higher the concentration and the higher/more solute has been dissolved in the solvent to make one cubic decimeter/ litre/1000cm3 solution.
Examples
2M sodium hydroxide means 2 moles of sodium hydroxide solute is dissolved in enough water to make one cubic decimeter/ litre/1000cm3 uniform solution mixture of sodium hydroxide and water.
0.02M sodium hydroxide means 0.02 moles of sodium hydroxide solute is dissolved in enough water to make one cubic decimeter/ litre/1000cm3 uniform solution mixture of sodium hydroxide and water.
“2M” is more concentrated than“0.02M”.
Preparation of molar solution
Procedure
Weigh accurately 4.0 g of sodium hydroxide pellets into a 250cm3 volumetric flask.
Using a wash bottle add about 200cm3 of distilled water.
Stopper the flask.
Shake vigorously for three minutes.
Remove the stopper for a second then continue to shake for about another two minutes until all the solid has dissolved.
Add more water slowly upto exactly the 250 cm3 mark.
Sample questions
1.Calculate the number of moles of sodium hydroxide pellets present in:
(i) 4.0 g.
Molar mass of NaOH = (23 + 16 + 1) = 40g
Moles = Mass => 4.0 = 0.1 / 1.0 x 10 -1 moles
Molar mass 40
(ii) 250 cm3 solution in the volumetric flask.
Moles in 250 cm3 = 0.1 / 1.0 x 10 -1 moles
(iii) one decimeter of solution
Method 1
Moles in decimeters = Molarity = Moles x 1000cm3/1dm3
Volume of solution
=> 1.0 x 10 -1 moles x 1000cm3 =
250cm3
= 0.4 M / 0.4 molesdm-3
Method 2
250cm3 solution contain 1.0 x 10 -1 moles
1000cm3 solution = Molarity contain 1000 x 1.0 x 10 -1 moles
250 cm3
= 0.4 M / 0.4 molesdm-3
Theoretical sample practice
1. Calculate the molarity of a solution containing:
(i) 4.0 g sodium hydroxide dissolved in 500cm3 solution
Molar mass of NaOH = (23 + 16 + 1) = 40g
Moles = Mass => 4.0 = 0.1 / 1.0 x 10 -1 moles
Molar mass 40
Method 1
Moles in decimeters = Molarity = Moles x 1000cm3/1dm3
Volume of solution
=> 1.0 x 10 -1 moles x 1000cm3
500cm3
= 0.2 M / 0.2 molesdm-3
Method 2
500 cm3 solution contain 1.0 x 10 -1 moles
1000cm3 solution = Molarity contain 1000 x 1.0 x 10 -1 moles
500 cm3
= 0.2 M / 0.2 molesdm-3
(ii) 5.3 g anhydrous sodium carbonate dissolved in 50cm3 solution
Molar mass of Na2CO3 = (23 x 2 + 12 + 16 x 3) = 106 g
Moles = Mass => 5.3 = 0.05 / 5. 0 x 10-2 moles
Molar mass 106
Method 1
Moles in decimeters = Molarity = Moles x 1000cm3/1dm3
Volume of solution
=> 1.0 moles x 1000cm3 =
50cm3
=1.0 M
Method 2
50 cm3 solution contain 5.0 x 10 -2 moles
1000cm3 solution = Molarity contain 1000 x 5.0 x 10 -2 moles
50 cm3
= 1.0M / 1.0 molesdm-3
(iii) 5.3 g hydrated sodium carbonate decahydrate dissolved in 50cm3 solution
Molar mass of Na2CO3.10H2O = (23 x 2 + 12 + 16 x 3 + 20 x 1 + 10 x 16) =286g
Moles = Mass => 5.3 = 0.0185 / 1.85 x 10 -2 moles
Molar mass 286
Method 1
Moles in decimeters = Molarity = Moles x 1000cm3/1dm3
Volume of solution
=> 1.85 x 10 -2 moles x 1000cm3 =
50cm3
= 0.37 M/0.37 molesdm-3
Method 2
50 cm3 solution contain 1.85 x 10 -2 moles
1000cm3 solution = Molarity contain 1000 x 1.85 x 10 -2 moles
50 cm3
= 3.7 x 10-1 M / 3.7 x 10-1 molesdm-3
(iv) 7.1 g of anhydrous sodium sulphate(VI)was dissolved in 20.0 cm3 solution. Calculate the molarity of the solution.
Method 1
20.0cm3 solution ->7.1 g
1000cm3 solution -> 1000 x 71 = 3550 g dm-3
20
Molar mass Na2SO4 = 142 g
Moles dm-3 = Molarity = Mass 3550 = 2.5 M/ molesdm-3
Molar mass 142
Method 2
Molar mass Na2SO4 = 142 g
Moles = Mass => 7.1 = 0.05 / 5.0 x 10 -2 moles
Molar mass 142
Method 2(a)
Moles in decimeters = Molarity = Moles x 1000cm3/1dm3
Volume of solution
=> 5.0 x 10 -2 moles x 1000cm3
20cm3
= 2.5 M/2.5 molesdm-3
Method 2(b)
20 cm3 solution contain 5.0 x 10 -2 moles
1000cm3 solution = Molarity contain 1000 x 5.0 x 10 -2 moles
20 cm3
= 2.5 M/2.5 molesdm-3
(iv) The density of sulphuric(VI) is 1.84gcm-3 Calculate the molarity of the acid.
Method 1
1.0cm3 solution ->1.84 g
1000cm3 solution -> 1000 x 1.84 = 1840 g dm-3
1
Molar mass H2SO4 = 98 g
Moles dm-3 = Molarity = Mass = 1840
Molar mass 98
= 18.7755 M/ molesdm-3
Method 2
Molar mass H2SO4 = 98 g
Moles = Mass => 1.84 = 0.0188 / 1.88 x 10 -2 moles
Molar mass 98
Method 2(a)
Moles in decimeters = Molarity = Moles x 1000cm3/1dm3
Volume of solution
=> 1.88 x 10 -2 moles x 1000cm3
1.0cm3
= 18.8M/18.8 molesdm-3
Method 2(b)
20 cm3 solution contain 1.88 x 10 -2 moles
1000cm3 solution = Molarity contain 1000 x 1.88 x 10 -2 moles
1.0 cm3
= 18.8M/18.8 molesdm-3
2. Calculate the mass of :
(i) 25 cm3 of 0.2M sodium hydroxide solution(Na =23.0.O =16.0, H=1.0)
Molar mass NaOH = 40g
Moles in 25 cm3 = Molarity x volume => 0.2 x 25 = 0.005/5.0 x 10-3moles
1000 1000
Mass of NaOH =Moles x molar mass = 5.0 x 10-3 x 40 = 0.2 g
(ii) 20 cm3 of 0.625 M sulphuric(VI)acid (S =32.0.O =16.0, H=1.0)
Molar mass H2SO4 = 98g
Moles in 20 cm3 = Molarity x volume=> 0.625 x 20 = 0.0125/1.25.0 x 10-3moles
1000 1000
Mass of H2SO4 =Moles x molar mass => 5.0 x 10-3 x 40 = 0.2 g
(iii) 1.0 cm3 of 2.5 M Nitric(V)acid (N =14.0.O =16.0, H=1.0)
Molar mass HNO3 = 63 g
Moles in 1 cm3 = Molarity x volume => 2.5 x 1 = 0.0025 / 2.5. x 10-3moles
1000 1000
Mass of HNO3 =Moles x molar mass => 2.5 x 10-3 x 40 = 0.1 g
3. Calculate the volume required to dissolve :
(a)(i) 0.25moles of sodium hydroxide solution to form a 0.8M solution
Volume (in cm3) = moles x 1000 => 0.25 x 1000 = 312.5cm3
Molarity 0.8
(ii) 100cm3 was added to the sodium hydroxide solution above. Calculate the concentration of the solution.
C1 x V1 = C2 x V2 where:
C1 = molarity/concentration before diluting/adding water
C2 = molarity/concentration after diluting/adding water
V1 = volume before diluting/adding water
V2 = volume after diluting/adding water
=> 0.8M x 312.5cm3 = C2 x (312.5 + 100)
C2 = 0.8M x 312.5cm3 = 0.6061M
412.5
(b)(ii) 0.01M solution containing 0.01moles of sodium hydroxide solution .
Volume (in cm3) = moles x 1000 => 0.01 x 1000 = 1000 cm3
Molarity 0.01
(ii) Determine the quantity of water which must be added to the sodium hydroxide solution above to form a 0.008M solution.
C1 x V1 = C2 x V2 where:
C1 = molarity/concentration before diluting/adding water
C2 = molarity/concentration after diluting/adding water
V1 = volume before diluting/adding water
V2 = volume after diluting/adding water
=> 0.01M x 1000 cm3 = 0.008 x V2
V2 = 0.01M x 1000cm3 = 1250cm3
0.008
Volume added = 1250 – 1000 = 250cm3
(c)Volumetric analysis/Titration
Volumetric analysis/Titration is the process of determining unknown concentration of one reactant from a known concentration and volume of another.
Reactions take place in simple mole ratio of reactants and products.
Knowing the concentration/ volume of one reactant, the other can be determined from the relationship:
M1V1 = M2V2 where:
n1 n2
M1 = Molarity of 1st reactant
M2 = Molarity of 2nd reactant
V1 = Volume of 1st reactant
V1 = Volume of 2nd reactant
n1 = number of moles of 1st reactant from stoichiometric equation
n2 = number of moles of 2nd reactant from stoichiometric equation
Examples
1.Calculate the molarity of MCO3 if 5.0cm3 of MCO3 react with 25.0cm3 of 0.5M hydrochloric acid.(C=12.0 ,O =16.0)
Stoichiometric equation:MCO3(s) + 2HCl(aq) -> MCl2(aq) + CO2(g) + H2O(l)
Method 1
M1V1 = M2V2 -> M1 x 5.0cm3 = 0.5M x 25.0cm3
n1 n2 1 2
=> M1 = 0.5 x 25.0 x1 = 1.25M / 1.25 moledm-3
5.0 x 2
Method 2
Moles of HCl used = molarity x volume
1000
=> 0.5 x 25.0 = 0.0125 /1.25 x 10-2moles
1000
Mole ratio MCO3 : HCl = 1:2
Moles MCO3 = 0.0125 /1.25 x 10-2moles = 0.00625 / 6.25 x 10-3 moles
2
Molarity MCO3 = moles x 1000 => 0.00625 / 6.25 x 10-3 x 1000
Volume 5
= 1.25M / 1.25 moledm-3
2. 2.0cm3 of 0.5M hydrochloric acid react with 0.1M of M2CO3. Calculate the volume of 0.1M M2CO3 used.
Stoichiometric equation:M2CO3 (aq) + 2HCl(aq) -> 2MCl (aq) + CO2(g) + H2O(l)
Method 1
M1V1 = M2V2 -> 0.5 x 2.0cm3 = 0.1M x V2 cm3
n1 n2 2 1
=> V2 = 0.5 x 2.0 x1 = 1.25M / 1.25 moledm-3
0.1 x 2
Method 2
Moles of HCl used = molarity x volume
1000
=> 0.5 x 2.0 = 0.0125 /1.25 x 10-2moles
1000
Mole ratio M2CO3 : HCl = 1:2
Moles M2CO3 = 0.0125 /1.25 x 10-2moles = 0.00625 / 6.25 x 10-3 moles
2
Molarity M2CO3 = moles x 1000 => 0.00625 / 6.25 x 10-3 x 1000
Volume 5
= 1.25M / 1.25 moledm-3
3. 5.0cm3 of 0.1M sodium iodide react with 0.1M of Lead(II)nitrate(V). Calculate(i) the volume of Lead(II)nitrate(V) used.
(ii)the mass of Lead(II)Iodide formed
(Pb=207.0, I =127.0)
Stoichiometric equation: 2NaI(aq) + Pb(NO3)2(aq) -> 2NaNO3(aq) + PbI2(s)
(i)Volume of Lead(II)nitrate(V) used
Method 1
M1V1 = M2V2 -> 5 x 0.1cm3 = 0.1M x V2 cm3
n1 n2 2 1
=> V2 = 0.1 x 5.0 x 1 = 1.25M / 1.25 moledm-3
0.1 x 2
Method 2
Moles of HCl used = molarity x volume
1000
=> 0.1 x 5.0 = 0.0125 /1.25 x 10-2moles
1000
Mole ratio M2CO3 : HCl = 1:2
Moles M2CO3 = 0.0125 /1.25 x 10-2moles = 0.00625 / 6.25 x 10-3 moles
2
Molarity M2CO3 = moles x 1000 => 0.00625 / 6.25 x 10-3 x 1000
Volume 5
= 1.25M / 1.25 moledm-3
4. 0.388g of a monobasic organic acid B required 46.5 cm3 of 0.095M sodium hydroxide for complete neutralization. Name and draw the structural formula of B
Moles of NaOH used = molarity x volume
1000
=> 0.095 x 46.5 = 0.0044175 /4.4175 x 10-3moles
1000
Mole ratio B: NaOH = 1:1
Moles B= 0.0044175 /4.4175 x 10-3moles
Molar mass B = mass => 0.388
moles 0.0044175 /4.4175 x 10-3moles
= 87.8324 gmole-1
X-COOH = 87.8324 where X is an alkyl group
X =87.8324- 42 = 42.8324=43
By elimination: CH3 = 15 CH3CH2 = 29 CH3CH2 CH2 = 43
Molecula formula : CH3CH2 CH2COOH
Molecule name : Butan-1-oic acid
Molecular structure
H H H O
H C C C C O H
H H H H
5. 10.5 g of an impure sample containing ammonium sulphate (VI) fertilizer was warmed with 250cm3 of o.8M sodium hydroxide solution.The excess of the alkali was neutralized by 85cm3 of 0.5M hydrochloric acid. Calculate the % of impurities in the ammonium sulphate (VI)fertilizer. (N=14.0,S=32.0,O=16.0, H=1.0)
Equation for neutralization
NaOH(aq) + HCl(aq) -> NaOH(aq) + H2O(l)
Mole ratio NaOH(aq):HCl(aq)= 1:1
Moles of HCl = Molarity x volume => 0.5 x 85 = 0.0425 moles
1000 1000
Excess moles of NaOH(aq)= 0.0425 moles
Equation for reaction with ammonium salt
2NaOH(aq) + (NH4) 2SO4(aq) -> Na 2SO4(aq) + 2NH3 (g)+ 2H2O(l)
Mole ratio NaOH(aq): (NH4) 2SO4(aq)= 2:1
Total moles of NaOH = Molarity x volume => 0.8 x 250 = 0.2 moles
1000 1000
Moles of NaOH that reacted with(NH4) 2SO4 = 0.2 – 0.0425 = 0.1575moles
Moles (NH4) 2SO4 = ½ x 0.1575moles = 0. 07875moles
Molar mass (NH4) 2SO4= 132 gmole-1
Mass of in impure sample = moles x molar mass =>0. 07875 x 132 = 10.395 g
Mass of impurities = 10.5 -10.395 = 0.105 g
% impurities = 0.105 x 100 = 1.0 %
10.5
Practically volumetric analysis involves titration.
Titration generally involves filling a burette with known/unknown concentration of a solution then adding the solution to unknown/known concentration of another solution in a conical flask until there is complete reaction. If the solutions used are both colourless, an indicator is added to the conical flask. When the reaction is over, a slight/little excess of burette contents change the colour of the indicator. This is called the end point.
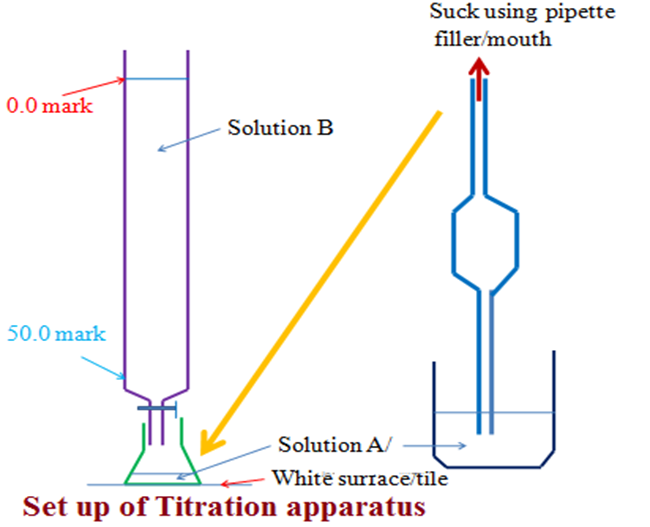
The titration process involve involves determination of titre. The titre is the volume of burette contents/reading before and after the end point. Burette contents/reading before titration is usually called the Initial burette reading. Burette contents/reading after titration is usually called the Final burette reading. The titre value is thus a sum of the Final less Initial burette readings.
To reduce errors, titration process should be repeated at least once more.
The results of titration are recorded in a titration table as below
Sample titration table
| Titration number | 1 | 2 | 3 |
| Final burette reading (cm3) | 20.0 | 20.0 | 20.0 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of solution used(cm3) | 20.0 | 20.0 | 20.0 |
As evidence of a titration actually done examining body requires the candidate to record their burette readings before and after the titration.
For KCSE candidates burette readings must be recorded in a titration table in the format provided by the Kenya National Examination Council.
As evidence of all titration actually done Kenya National Examination Council require the candidate to record their burette readings before and after the titration to complete the titration table in the format provided.
Calculate the average volume of solution used
24.0 + 24.0 + 24.0 = 24.0 cm3
3
As evidence of understanding the degree of accuracy of burettes , all readings must be recorded to a decimal point.
As evidence of accuracy in carrying the out the titration , candidates value should be within 0.2 of the school value .
The school value is the teachers readings presented to the examining body/council based on the concentrations of the solutions s/he presented to her/his candidates.
Bonus mark is awarded for averaged reading within 0.1 school value as Final answer.
Calculations involved after the titration require candidates thorough practical and theoretical practice mastery on the:
(i)relationship among the mole, molar mass, mole ratios, concentration, molarity.
(ii) mathematical application of 1st principles.
Very useful information which candidates forget appears usually in the beginning of the question paper as:
“You are provided with…”
All calculation must be to the 4th decimal point unless they divide fully to a lesser decimal point.
Candidates are expected to use a non programmable scientific calculator.
(a)Sample Titration Practice 1 (Simple Titration)
You are provided with:
0.1M sodium hydroxide solution A
Hydrochloric acid solution B
You are required to determine the concentration of solution B in moles per litre.
Procedure
Fill the burette with solution B. Pipette 25.0cm3 of solution A into a conical flask. Titrate solution A with solution B using phenolphthalein indicator to complete the titration table 1
Sample results Titration table 1
| Titration number | 1 | 2 | 3 |
| Final burette reading (cm3) | 20.0 | 20.0 | 20.0 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of solution B used(cm3) | 20.0 | 20.0 | 20.0 |
Sample worked questions
1. Calculate the average volume of solution B used
Average titre = Titre 1 + Titre 2 +Titre 3 => ( 20.0 +20.0 +20.0 ) = 20.0cm3
3 3
2. How many moles of:
(i)solution A were present in 25cm3 solution.
Moles of solution A = Molarity x volume = 0.1 x 25 = 2.5 x 10-3 moles
1000 1000
(ii)solution B were present in the average volume.
Chemical equation: NaOH(aq) + HCl(aq) -> NaCl(aq) + H2O(l)
Mole ratio 1:1 => Moles of A = Moles of B = 2.5 x 10-3 moles
(iii) solution B in moles per litre.
Moles of B per litre = moles x 1000 = 2.5 x 10-3 x 1000 = 0.1M
Volume 20
(b)Sample Titration Practice 2 (Redox Titration)
You are provided with:
Acidified Potassium manganate(VII) solution A
0.1M of an iron (II)salt solution B
8.5g of ammonium iron(II)sulphate(VI) crystals(NH4)2 SO4FeSO4.xH2O solid C
You are required to
(i)standardize acidified potassium manganate(VII)
(ii)determine the value of x in the formula (NH4)2 SO4FeSO4.xH2O.
Procedure 1
Fill the burette with solution A. Pipette 25.0cm3 of solution B into a conical flask. Titrate solution A with solution B until a pink colour just appears.
Record your results to complete table 1.
Table 1:Sample results
| Titration number | 1 | 2 | 3 |
| Final burette reading (cm3) | 20.0 | 20.0 | 20.0 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of solution A used(cm3) | 20.0 | 20.0 | 20.0 |
Sample worked questions
1. Calculate the average volume of solution A used
Average titre = Titre 1 + Titre 2 +Titre 3 => ( 20.0 +20.0 +20.0 ) = 20.0cm3
3 3
2. How many moles of:
(i)solution B were present in 25cm3 solution.
Moles of solution A = Molarity x volume = 0.1 x 25 = 2.5 x 10-3 moles
1000 1000
(ii)solution A were present in the average volume. Assume one mole of B react with five moles of B
Mole ratio A : B = 1:5
=> Moles of A = Moles of B = 2.5 x 10-3 moles = 5.0 x 10 -4 moles
5 5
(iii) solution B in moles per litre.
Moles of B per litre = moles x 1000 = 2.5 x 10-3 x 1000
Volume 20
= 0.025 M /moles per litre /moles l-1
Procedure 2
Place all the solid C into the 250cm3 volumetric flask carefully. Add about 200cm3 of distilled water. Shake to dissolve. Make up to the 250cm3 of solution by adding more distilled water. Label this solution C. Pipette 25cm3 of solution C into a conical flask, Titrate solution C with solution A until a permanent pink colour just appears. Complete table 2.
Table 2:Sample results
| Titration number | 1 | 2 | 3 |
| Final burette reading (cm3) | 20.0 | 20.0 | 20.0 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of solution A used(cm3) | 20.0 | 20.0 | 20.0 |
Sample worked questions
1. Calculate the average volume of solution A used
Average titre = Titre 1 + Titre 2 +Titre 3 => ( 20.0 +20.0 +20.0 ) = 20.0cm3
3 3
2. How many moles of:
(i)solution A were present inin the average titre.
Moles of solution A = Molarity x volume = 0.025 x 20 = 5.0 x 10-4 moles
1000 1000
(ii)solution C in 25cm3 solution given the equation for the reaction:
MnO4– (aq) + 8H+(aq) + 5Fe2+ (aq) -> Mn2+(aq) + 5Fe3+(aq) + 4H2O(l)
Mole ratio MnO4– (aq): 5Fe2+ (aq) = 1:5 => Moles of 5Fe2+ (aq) = Moles of MnO4– (aq) = 5.0 x 10-4 moles = 1.0 x 10 -4 moles
5 5
(iii) solution B in 250cm3.
Moles of B per litre = moles x 250 = 1.0 x 10 -4 x 250 = 1.0 x 10 -3 moles Volume 25
3. Calculate the molar mass of solid C and hence the value of x in the chemical formula (NH4)2SO4FeSO4.xH2O.
(N=14.0, S=32.0, Fe=56.0, H=1.0 O=16.0)
Molar mass = mass perlitre = 8.5 = 8500 g
Moles per litre 1.0 x 10 -3 moles
NH4)2SO4FeSO4.xH2O = 8500
284 + 18x =8500
8500 – 284 = 8216 = 18x = 454.4444
18 18
x = 454 (whole number)
(c)Sample Titration Practice 3 (Back titration)
You are provided with:
(i)an impure calcium carbonate labeled M
(ii)Hydrochloric acid labeled solution N
(iii)solution L containing 20g per litre sodium hydroxide.
You are required to determine the concentration of N in moles per litre and the % of calcium carbonate in mixture M.
Procedure 1
Pipette 25.0cm3 of solution L into a conical flask. Add 2-3 drops of phenolphthalein indicator. Titrate with dilute hydrochloric acid solution N and record your results in table 1(4mark)
Sample Table 1
| 1 | 2 | 3 | |
| Final burette reading (cm3) | 6.5 | 6.5 | 6.5 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of N used (cm3) | 6.5 | 6.5 | 6.5 |
Sample questions
(a) Calculate the average volume of solution N used
6.5 + 6.5 + 6.5 = 6.5 cm3
3
(b) How many moles of sodium hydroxide are contained in 25cm3of solution L
Molar mass NaOH =40g
Molarity of L = mass per litre => 20 = 0.5M
Molar mass NaOH 40
Moles NaOH in 25cm3 = molarity x volume => 0.5M x 25cm3 = 0.0125 moles
1000 1000
(c)Calculate:
(i)the number of moles of hydrochloric acidthat react with sodium hydroxide in (b)above.
Mole ratio NaOH : HCl from stoichiometric equation= 1:1
Moles HCl =Moles NaOH => 0.0125 moles
(ii)the molarity of hydrochloric acid solution N.
Molarity = moles x 1000 => 0.0125 moles x 1000 =1.9231M/moledm-3
6.5 6.5
Procedure 2
Place the 4.0 g of M provided into a conical flask and add 25.0cm3 of the dilute hydrochloric acid to it using a clean pipette. Swirl the contents of the flask vigorously until effervescence stop.Using a 100ml measuring cylinder add 175cm3 distilled waterto make up the solution up to 200cm3.Label this solution K.Using a clean pipettetransfer 25.0cm3 of the solution into a clean conical flask and titrate with solution L from the burette using 2-3 drops of methyl orange indicator.Record your observations in table 2.
Sample Table 2
| 1 | 2 | 3 | |
| Final burette reading (cm3) | 24.5 | 24.5 | 24.5 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of N used (cm3) | 24.5 | 24.5 | 24.5 |
Sample calculations
(a)Calculate the average volume of solution L used(1mk)
24.5 + 24.5 + 24.5 = 24.5cm3
3
(b)How many moles of sodium hydroxide are present in the average volume of solution L used?
Moles = molarity x average burette volume => 0.5 x 24.5
1000 1000
= 0.01225 /1.225 x 10-2 moles
(c) How many moles of hydrochloric acid are present in the original 200cm3 of solution K?
Mole ratio NaOH: HCl = 1:1 => moles of HCl = 0.01225 /1.225 x 10-2 moles
Moles in 200cm3 = 200cm3 x 0.01225 /1.225 x 10-2moles
25cm3(volume pipetted)
=0.49 /4.9 x 10-1moles
(d)How many moles of hydrochloric acid were contained in original 25 cm3 solution N used
Original moles = Original molarity x pipetted volume =>
1000cm3
1.9231M/moledm-3 x 25 = 0.04807/4.807 x 10-2 moles
1000
(e)How many moles of hydrochloric acid were used to react with calcium carbonate present?
Moles that reacted = original moles –moles in average titre =>
0.04807/4.807 x 10-2moles – 0.01225 /1.225 x 10-2moles
= 0.03582/3.582 x 10 -2moles
(f)Write the equation for the reaction between calcium carbonate and hydrochloric acid.
CaCO3(s) + 2HCl(aq) -> CaCl2(aq) + CO2(g) + H2O(l)
(g)Calculate the number of moles of calcium carbonate that reacted with hydrochloric acid.
From the equation CaCO3(s):2HCl(aq) = 1:2
=> Moles CaCO3(s) = 1/2moles HCl
= 1/2 x 0.03582/3.582 x 10 -2 moles
= 0.01791 /1.791 x 10-2moles
(h)Calculate the mass of calcium carbonate in 4.0g of mixture M (Ca=40.0,O = 16.0,C=12.0)
Molar mass CaCO3 = 100g
Mass CaCO3 = moles x molar mass => 0.01791 /1.791 x 10-2moles x 100g
= 1.791g
(i)Determine the % of calcium carbonate present in the mixture
% CaCO3 = mass of pure x 100% => 1.791g x 100% = 44.775%
Mass of impure 4.0
(d)Sample titration practice 4 (Multiple titration)
You are provided with:
(i)sodium L containing 5.0g per litre of a dibasic organic acid H2X.2H2O.
(ii)solution M which is acidified potassium manganate(VII)
(iii)solution N a mixture of sodium ethanedioate and ethanedioic acid
(iv)0.1M sodium hydroxide solution P
(v)1.0M sulphuric(VI)
You are required to:
(i)standardize solution M using solution L
(ii)use standardized solution M and solution P to determine the % of sodium ethanedioate in the mixture.
Procedure 1
Fill the burette with solution M. Pipette 25.0cm3 of solution L into a conical flask. Heat this solution to about 70oC(but not to boil).Titrate the hot solution L with solution M until a permanent pink colour just appears .Shake thoroughly during the titration. Repeat this procedure to complete table 1.
Sample Table 1
| 1 | 2 | 3 | |
| Final burette reading (cm3) | 24.0 | 24.0 | 24.0 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of N used (cm3) | 24.0 | 24.0 | 24.0 |
Sample calculations
(a)Calculate the average volume of solution L used (1mk)
24.0 + 24.0 + 24.0 = 24.0cm3
3
(b)Given that the concentration of the dibasic acid is 0.05molesdm-3.determine the value of x in the formula H2X.2H2O (H=1.0,O=16.0)
Molar mass H2X.2H2O= mass per litre => 5.0g/litre = 100g
Moles/litre 0.05molesdm-3
H2X.2H2O =100
X = 100 – ((2 x1) + 2 x (2 x1) + (2 x 16) => 100 – 34 = 66
(c) Calculate the number of moles of the dibasic acid H2X.2H2O.
Moles = molarity x pipette volume => 0.5 x 25 = 0.0125/1.25 x10 -2 moles
1000 1000
(d)Given the mole ratio manganate(VII)(MnO4–): acid H2X is 2:5, calculate the number of moles of manganate(VII) (MnO4–) in the average titre.
Moles H2X = 2/5 moles of MnO4–
=> 2/5 x 0.0125/1.25 x10 -2 moles
= 0.005/5.0 x 10 -3moles
(e)Calculate the concentration of the manganate(VII)(MnO4–) in moles per litre.
Moles per litre/molarity = moles x 1000
average burette volume
=>0.005/5.0 x 10 -3moles x 1000 = 0.2083 molesl-1/M
24.0
Procedure 2
With solution M still in the burette ,pipette 25.0cm3 of solution N into a conical flask. Heat the conical flask containing solution N to about 70oC.Titrate while hot with solution M.Repeat the experiment to complete table 2.
Sample Table 2
| 1 | 2 | 3 | |
| Final burette reading (cm3) | 12.5 | 12.5 | 12.5 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of N used (cm3) | 12.5 | 12.5 | 12.5 |
Sample calculations
(a)Calculate the average volume of solution L used (1mk)
12.5 + 12.5 + 12.5 =12.5cm3
3
(b)Calculations:
(i)How many moles of manganate(VII)ions are contained in the average volume of solution M used?
Moles = molarity of solution M x average burette volume
1000
=> 0.2083 molesl-1/ M x 12.5 = 0.0026 / 2.5 x 10-3 moles
1000
(ii)The reaction between manganate(VII)ions and ethanedioate ions that reacted with is as in the equation:
2MnO4– (aq) + 5C2O42- (aq) + 16H+ (aq) -> 2Mn2+(aq) + 10CO2(g) + 8H2O(l)
Calculate the number of moles of ethanedioate ions that reacted with manganate (VII) ions in the average volume of solution M.
From the stoichiometric equation,mole ratio MnO4– (aq): C2O42- (aq) = 2:5
=> moles C2O42- = 5/2 moles MnO4– => 5/2 x 0.0026 / 2.5 x 10-3 moles
= 0.0065 /6.5 x10-3 moles
(iii)Calculate the number of moles of ethanedioate ions contained in 250cm3 solution N.
25cm3 pipette volume -> 0.0065 /6.5 x10-3 moles
250cm3 ->
0.0065 /6.5 x10-3 moles x 250 = 0.065 / 6.5 x10-2 moles
25
Procedure 3
Remove solution M from the burette and rinse it with distilled water. Fill the burette with sodium hydroxide solution P. Pipette 25cm3 of solution N into a conical flask and add 2-3 drops of phenolphthalein indicator. Titrate this solution N with solution P from the burette. Repeat the procedure to complete table 3.
Sample Table 2
| 1 | 2 | 3 | |
| Final burette reading (cm3) | 24.9 | 24.9 | 24.9 |
| Initial burette reading (cm3) | 0.0 | 0.0 | 0.0 |
| Volume of N used (cm3) | 24.9 | 24.9 | 24.9 |
Sample calculations
(a)Calculate the average volume of solution L used (1mk)
24.9 + 24.9 + 24.9 = 24.9 cm3
3
(b)Calculations:
(i)How many moles of sodium hydroxide solution P were contained in the average volume?
Moles = molarity of solution P x average burette volume
1000
=> 0.1 molesl-1 x 24.9 = 0.00249 / 2.49 x 10-3 moles
1000
(ii)Given that NaOH solution P reacted with the ethanedioate ions from the acid only and the equation for the reaction is:
2NaOH(aq) + H2C2O4 (aq) -> Na2C2O4(g) + 2H2O(l)
Calculate the number of moles of ethanedioic acid that were used in the reaction
From the stoichiometric equation,mole ratio NaOH(aq): H2C2O4 (aq) = 2:1
=> moles H2C2O4 = 1/2 moles NaOH => 1/2 x 0.00249 / 2.49 x 10-3 moles
= 0.001245/1.245 x10-3 moles.
(iii)How many moles of ethanedioic acid were contained in 250cm3 of solution N?
25cm3 pipette volume -> 0.001245/1.245 x10-3 moles
250cm3 ->
0.001245/1.245 x10-3 moles x 250 = 0.01245/1.245 x10-2 moles
25
(iii)Determine the % by mass of sodium ethanedioate in the micture (H= 1.0,O=16.0,C=12.0 and total mass of mixture =2.0 g in 250cm3 solution)
Molar mass H2C2O4 = 90.0g
Mass of H2C2O4 in 250cm3 = moles in 250cm3 x molar mass H2C2O4
=>0.01245/1.245 x10-2 moles x 90.0
= 1.1205g
% by mass of sodium ethanedioate
=(Mass of mixture – mass of H2C2O4) x 100%
Mass of mixture
=> 2.0 – 1.1205 g = 43.975%
2.0
Note
(i) L is 0.05M Oxalic acid
(ii) M is 0.01M KMnO4
(iii) N is 0.03M oxalic acid(without sodium oxalate)
Practice example 5.(Determining equation for a reaction)
You are provided with
-0.1M hydrochloric acid solution A
-0.5M sodium hydroxide solution B
You are to determine the equation for thereaction between solution A and B
Procedure
Fill the burette with solution A.Using a pipette and pipette filler transfer 25.0cm3 of solution B into a conical flask.Add 2-3 drops of phenolphthalein indicator.Run solution A into solution B until a permanent pink colour just appears.Record your results in Table 1.Repeat the experiment to obtain three concordant results to complete Table 1
Table 1(Sample results)
| Titration | 1 | 2 | 3 |
| Final volume(cm3) | 12.5 | 25.0 | 37.5 |
| Initial volume(cm3) | 0.0 | 12.5 | 25.0 |
| Volume of solution A used(cm3) | 12.5 | 12.5 | 12.5 |
Sample questions
Calculate the average volume of solution A used.
12.5+12.5+12.5 = 12.5cm3
3
Theoretical Practice examples
1. 1.0g of dibasic acid HOOC(CH2)xCOOH was dissolved in 250cm3 solution. 25.0 cm3 of this solution reacted with 30.0cm3 of 0.06M sodium hydroxide solution. Calculate the value of x in HOOC(CH2)xCOOH. (C=12.0,H=1.0,O=16.)
Chemical equation
2NaOH(aq) + H2X(aq) -> Na2X (aq) + 2H2O(aq)
Mole ratio NaOH(aq) :H2X(aq) = 2:1
Method 1
Ma Va = na => Ma x 25.0 = 1 => Ma =0.06 x 30.0 x1
Mb Vb = nb 0.06 x 30.0 2 25.0 x 2
Molarity of acid = 0.036M/Mole l-1
Mass of acid per lite = 1.0 x1000 = 4.0 g/l
250
0.036M/ Mole l-1 -> 4.0 g /l
1 mole= molar mass of HOOC(CH2)xCOOH = 4.0 x 1 = 111.1111 g
0.036
Molar mass (CH2)x = 111.1111 – (HOOCCOOH = 90.0) = 21.1111
(CH2)x = 14x = 21.1111 = 1.5 = 1 (whole number)
14
Method 2
Moles of sodium hydroxide = Molarity x volume = 0.06 x 30 = 1.8 x 10 -3moles
1000
Moles of Hydrochloric acid = 1/2 x 1.8 x 10 -3moles = 9.0 x10 -4moles
Molarity of Hydrochloric acid = moles x 1000 = 9.0 x10 -4moles x1000
Volume 25
Molarity of acid = 0.036M/Mole l-1
Mass of acid per lite = 1.0 x1000 = 4.0 g/l
250
0.036M/ Mole l-1 -> 4.0 g /l
1 mole= molar mass of HOOC(CH2)xCOOH = 4.0 x 1 = 111.1111 g
0.036
Molar mass (CH2)x = 111.1111 – (HOOCCOOH = 90.0) = 21.1111
(CH2)x = 14x = 21.1111 = 1.5 = 1 (whole number)
14
2. 20.0cm3 of 0.05 M acidified potassium manganate(VII)solution oxidized 25.0cm3 of Fe2+(aq) ions in 40.0g/l of impure Iron (II)sulphate(VI) to Fe3+(aq) ions. Calculate the percentage impurities in the Iron (II)sulphate(VI).
MnO4– (aq) + 8H+(aq)+ 5Fe2+(aq)-> 5Fe3+(aq) + Mn2+(aq) + 4H2O(aq)
Fe=56.0,S= 32.0, O=16.0).
Moles of MnO4– (aq) = Molarity x volume = 0.05 x 20.0 = 0.001 Moles
1000 1000
Mole ratio MnO4– (aq): 5Fe2+(aq)= 1:5
Moles 5Fe2+(aq) = 5 x0.001 = 0.005 Moles
Moles of 5Fe2+(aq) per litre/molarity = Moles x 1000 = 0005 x 1000
Volume 25.0
= 0.2 M/ Moles/litre
Molar mass =FeSO4=152 g
Mass of in the mixture = Moles x molar mass => 0.2 x 152 = 30.4 g
Mass of impurity = 40.0 – 30.4 =9.6 g
% impurity = 9.6 g x100 = 24.0 % impurity
40.0
3.9.7 g of a mixture of Potassium hydroxide and Potassium chloride was dissolved to make one litre solution.20.0cm3 of this solution required 25.0cm3 of 0.12M hydrochloric acid for completed neutralization. Calculate the percentage by mass of Potassium chloride.(K=39.0,Cl= 35.5)
Chemical equation
KOH(aq) + HCl(aq) -> KCl(aq) + H2O(l)
Moles of HCl = Molarity x volume => 0.12 x 25.0 = 0.003/3.0 x 10 -3 moles
1000 1000
Mole ratio KOH(aq) : HCl(aq) -= 1:1
Moles KOH =0.003/3.0 x 10 -3 moles
Method 1
Molar mass KOH =56.0g
Mass KOH in 25cm3 =0.003/3.0 x 10 -3 moles x56.0 = 0.168g
Mass KOH in 1000cm3/1 litre = 0.168 x1000= 8.4 g/l
20
Mass of KCl = 9.7g – 8.4g = 1.3 g
% of KCl = 1.3 x 100 = 13.4021%
9.7
Method 2
Moles KOH in 1000cm3 /1 litre = Moles in 20cm3 x 1000 =>0.003 x 1000
20 20
=0.15M/Moles /litre
Molar mass KOH =56.0g
Mass KOH in 1000/1 litre = 0.15M/Moles /litre x 56.0 = 8.4g/l
Mass of KCl = 9.7g – 8.4g = 1.3 g
% of KCl = 1.3 x 100 = 13.4021%
9.7
4.A certain carbonate, GCO3, reacts with dilute hydrochloric acid according to the equation given below:
GCO3(s) + 2HCl(aq) -> GCl2 (aq) + CO2 (g) + H2O(l)
If 1 g of the carbonate reacts completely with 20 cm3 of 1 M hydrochloric acid ,calculate the relative atomic mass of G (C = 12.0 = 16.0)
Moles of HCl = Molarity x volume=> 1 x20 = 0.02 moles
1000 1000
Mole ratio HCl; GCO3 = 2:1
Moles of GCO3= 0.02 moles = 0.01moles
2
Molar mass of GCO3 = mass => 1 = 100 g
moles 0.01moles
G= GCO3 – CO3 =>100g – (12+ 16 x3 = 60) = 40(no units)
5. 46.0g of a metal carbonate MCO3 was dissolved 160cm3 of 0.1M excess hydrochloric acid and the resultant solution diluted to one litre.25.0cm3 of this solution required 20.0cm3 of 0.1M sodium hydroxide solution for complete neutralization. Calculate the atomic mass of ‘M’
Equation
Chemical equation
NaOH(aq) + HCl(aq) -> KCl(aq) + H2O(l)
Moles of NaOH = Molarity x volume=> 0.1 x20 = 0.002 moles
1000 1000
Mole ratio HCl; NaOH = 1:1
Excess moles of HCl = 0.002 moles
25cm3 -> 0.002 moles
1000cm3 -> 1000 x 0.002 = 0.08moles
25cm3
Original moles of HCl = Molarity x volume => 1M x 1litre = 1.0 moles
Moles of HCl reacted with MCO3 = 1.0 – 0.08 moles = 0.92moles
Chemical equation
MCO3(s) + 2HCl(aq) -> MCl2 (aq) + CO2 (g) + H2O(l)
Mole ratio MCO3(s) : HCl(aq) =1:2
Moles of MCO3 = 0.92moles => 0.46moles
2
Molar mass of MCO3= mass => 46g = 100 g
moles 0.46moles
M= MCO3 – CO3 =>100g – (12+ 16 x3 = 60) = 40
6. 25.0cm3 of a mixture of Fe2+ and Fe3+ ions in an aqueous salt was acidified with sulphuric(VI)acid then titrated against potassium manganate(VI).The salt required 15cm3 ofe0.02M potassium manganate(VI) for complete reaction.
A second 25cm3 portion of the Fe2+ and Fe3+ ion salt was reduced by Zinc then titrated against the same concentration of potassium manganate(VI).19.0cm3 of potassium manganate(VI)solution was used for complete reaction. Calculate the concentration of Fe2+ and Fe3+ ion in the solution on moles per litre.
Mole ratio Fe2+ :Mn04– = 5:1
Moles Mn04– used = 0.02 x 15 = 3.0 x 10-4 moles
1000
Moles Fe2+ = 3.0 x 10-4 moles = 6.0 x 10-5 moles
5
Molarity of Fe2+ = 6.0 x 10-4 moles x 1000 = 2.4 x 10-3 moles l-1
25
Since Zinc reduces Fe3+ to Fe2+ in the mixture:
Moles Mn04– that reacted with all Fe2+= 0.02 x 19 = 3.8 x 10-4 moles
1000
Moles of all Fe2+ = 3.8 x 10-4 moles = 7.6 x 10-5 moles
5
Moles of Fe3+ = 3.8 x 10-4 – 6.0 x 10-5 = 1.6 x 10-5 moles
Molarity of Fe3+ = 1.6 x 10-5 moles x 1000 = 4.0 x 10-4 moles l-1
