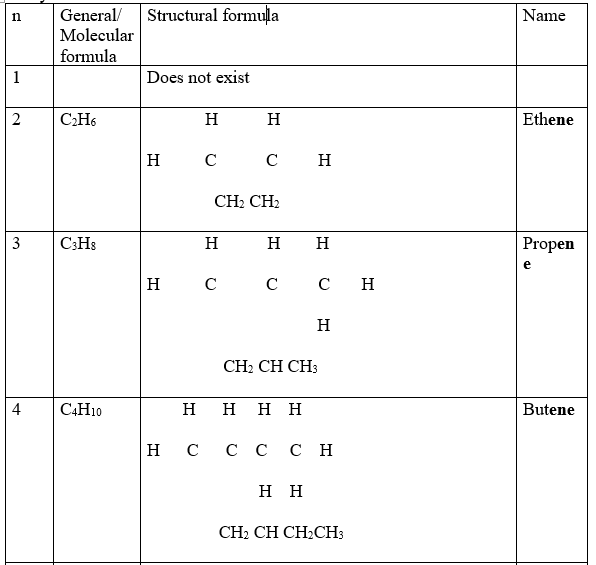
(a) Nomenclature/Naming of Alkenes
These are hydrocarbons with a general formula CnH2n and C C double bond as the functional group . n is the number of Carbon atoms in the molecule.
The carbon atoms are linked by at least one double bond to each other and single bonds to hydrogen atoms.
They include:
Note
1.Since carbon is tetravalent ,each atom of carbon in the alkene MUST always be bonded using four covalent bond /four shared pairs of electrons including at the double bond.
2.Since Hydrogen is monovalent ,each atom of hydrogen in the alkene MUST always be bonded using one covalent bond/one shared pair of electrons.
3.One member of the alkene ,like alkanes,differ from the next/previous by a CH2 group.They also form a homologous series.
e.g
Propene differ from ethene by one carbon and two Hydrogen atoms from ethene. 4.A homologous series of alkenes like that of alkanes:
(i) differ by a CH2 group from the next /previous consecutively
(ii)have similar chemical properties
(iii)have similar chemical formula represented by the general formula CnH2n
(iv)the physical properties also show steady gradual change
5.The = C= C = double bond in alkene is the functional group. A functional group is the reacting site of a molecule/compound.
6. The = C= C = double bond in alkene can easily be broken to accommodate more two more monovalent atoms. The = C= C = double bond in alkenes make it thus unsaturated.
7. An unsaturated hydrocarbon is one with a double =C=C= or triple – C C – carbon bonds in their molecular structure. Unsaturated hydrocarbon easily reacts to be saturated.
8.A saturated hydrocarbon is one without a double =C=C= or triple – C C – carbon bonds in their molecular structure.
Most of the reactions of alkenes take place at the = C = C =bond.
(b)Isomers of alkenes
Isomers are alkenes lie alkanes have the same molecular general formula but different molecular structural formula.
Ethene and propene do not form isomers. Isomers of alkenes are also named by using the IUPAC(International Union of Pure and Applied Chemistry) system of nomenclature/naming.
The IUPAC system of nomenclatureof naming alkenesuses the following basic rules/guidelines:
1.Identify the longest continuous/straight carbon chain which contains the =C = C= double bond get/determine the parent alkene.
2.Number the longest chain form the end of the chain which contains the =C = C= double bond so he =C = C= double bond lowest number possible.
3 Indicate the positions by splitting “alk-positions-ene” e.g. but-2-ene, pent-1,3-diene.
4.The position indicated must be for the carbon atom at the lower position in the =C = C= double bond.i.e
But-2-ene means the double =C = C= is between Carbon “2”and “3”
Pent-1,3-diene means there are two double bond one between carbon “1” and “2”and another between carbon “3” and “4”
5. Determine the position, number and type of branches. Name them as methyl, ethyl, propyl e.tc. according to the number of alkyl carbon chains attached to the alkene. Name them fluoro-,chloro-,bromo-,iodo- if they are halogens
6.Use prefix di-,tri-,tetra-,penta-,hexa- to show the number of double C = C bonds and branches attached to the alkene.
7.Position isomers can be formed when the=C = C= double bond is shifted between carbon atoms e.g.
But-2-ene means the double =C = C= is between Carbon “2”and “3”
But-1-ene means the double =C = C= is between Carbon “1”and “2”
Both But-1-ene and But-2-ene are position isomers of Butene
8.Position isomers are molecules/compounds having the same general formular but different position of the functional group.i.e.
Butene has the molecular/general formular C4H8 position but can form both But-1-ene and But-2-ene as position isomers.
9. Like alkanes ,an alkyl group can be attached to the alkene. Chain/branch isomers are thus formed.
10.Chain/branch isomers are molecules/compounds having the same general formula but different structural formula e.g
Butene and 2-methyl propene both have the same general formualr but different branching chain.
Practice on IUPAC nomenclature of alkenes
Name the following isomers of alkene
H H H H
H C C C C H But-1-ene
H H
H H H H
H C C C C H But-2-ene
H H
H H H H H H
H C C C C C C H 4-methylhex-1-ene
H H H
H C H
H
H
H C H
H H H H H
H C C C C C C H 4,4-dimethylhex-1-ene
H H H
H C H
H
3. H
H C H
H H H H
H C C C C C H 4,4-dimethylpent -1- ene
H H
H C H
H
4. H
H C H
H H H H
H C C C C C H 5,5-dimethylhex-2- ene
H C H H H
H C H
H
H
5. H
H C H
H H H
H C C C C H 2,2-dimethylbut -2- ene
H H
H C H
H
8.H2C CHCH2 CH2 CH3 pent -1- ene
9.H2C C(CH3)CH2 CH2 CH3 2-methylpent -1- ene
10.H2C C(CH3)C(CH3)2 CH2 CH3 2,3,3-trimethylpent -1- ene
11.H2C C(CH3)C(CH3)2 C(CH3)2 CH3 2,3,3,4,4-pentamethylpent -1- ene
12.H3C C(CH3)C(CH3) C(CH3)2 CH3 2,3,4,4-tetramethylpent -2- ene
13. H2C C(CH3)C(CH3) C(CH3) CH3 2,3,4-trimethylpent -1,3- diene
14. H2C CBrCBr CBr CH3 2,3,4-tribromopent -1,3- diene
15. H2C CHCH CH2 But -1,3- diene
16. Br2C CBrCBr CBr2 1,1,2,3,4,4-hexabromobut -1,3- diene
17. I2C CICI CI2 1,1,2,3,4,4-hexaiodobut -1,3- diene
18. H2C C(CH3)C(CH3) CH2 2,3-dimethylbut -1,3- diene
(c)Occurrence and extraction
At indusrial level,alkenes are obtained from the cracking of alkanes.Cracking is the process of breaking long chain alkanes to smaller/shorter alkanes, an alkene and hydrogen gas at high temperatures.
Cracking is a major source of useful hydrogen gas for manufacture of ammonia/nitric(V)acid/HCl i.e.
Long chain alkane -> smaller/shorter alkane + Alkene + Hydrogen gas
Examples
1.When irradiated with high energy radiation,Propane undergo cracking to form methane gas, ethene and hydrogen gas.
Chemical equation
CH3CH2CH3 (g) -> CH4(g) + CH2=CH2(g) + H2(g)
2.Octane undergo cracking to form hydrogen gas, butene and butane gases
Chemical equation
CH3(CH2) 6 CH3 (g) -> CH3CH2CH2CH3(g) + CH3 CH2CH=CH2(g) + H2(g)
(d)School laboratory preparation of alkenes
In a school laboratory, alkenes may be prepared from dehydration of alkanols using:
(i) concentrated sulphuric(VI)acid(H2SO4).
(a) aluminium(III)oxide(Al2O3) i.e
Alkanol –Conc. H2SO4 –> Alkene + Water
Alkanol –Al2O3 –> Alkene + Water e.g.
1.(a)At about 180oC,concentrated sulphuric(VI)acid dehydrates/removes water from ethanol to form ethene.
The gas produced contain traces of carbon(IV)oxide and sulphur(IV)oxide gas as impurities.
It is thus passed through concentrated sodium/potassium hydroxide solution to remove the impurities.
Chemical equation
CH3CH2OH (l) –conc H2SO4/180oC–> CH2=CH2(g) + H2O(l)
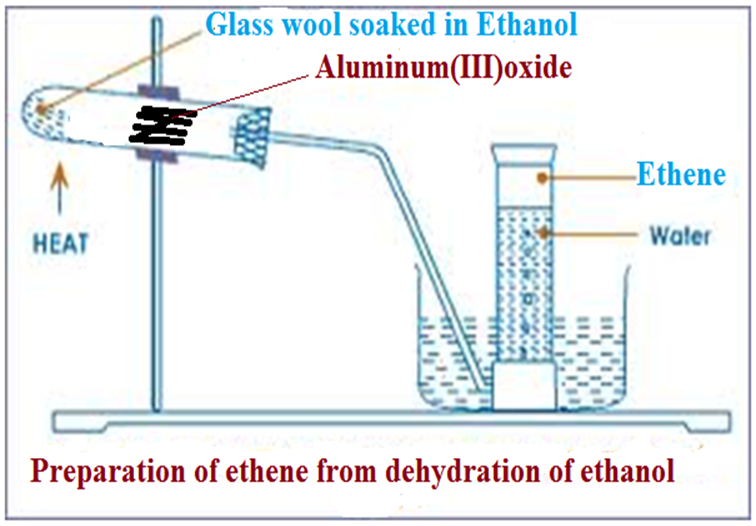
(b)On heating strongly aluminium(III)oxide(Al2O3),it dehydrates/removes water from ethanol to form ethene.
Ethanol vapour passes through the hot aluminium (III) oxide which catalyses the dehydration.
Activated aluminium(III)oxide has a very high affinity for water molecules/elements of water and thus dehydrates/ removes water from ethanol to form ethene.
Chemical equation
CH3CH2OH (l) –(Al2O3/strong heat–> CH2=CH2(g) + H2O(l)
2(a) Propan-1-ol and Propan-2-ol(position isomers of propanol) are dehydrated by conc H2SO4 at about 180oC to propene(propene has no position isomers).
Chemical equation
CH3CH2 CH2OH (l) — conc H2SO4/180oC –> CH3CH2=CH2(g) + H2O(l)
Propan-1-ol Prop-1-ene
CH3CHOH CH3 (l) — conc H2SO4/180oC –> CH3CH2=CH2(g) + H2O(l)
Propan-2-ol Prop-1-ene
(b) Propan-1-ol and Propan-2-ol(position isomers of propanol) are dehydrated by heating strongly aluminium(III)oxide(Al2O3) form propene
Chemical equation
CH3CH2 CH2OH (l) — Heat/Al2O3 –> CH3CH2=CH2(g) + H2O(l)
Propan-1-ol Prop-1-ene
CH3CHOH CH3 (l) — Heat/Al2O3 –> CH3CH2=CH2(g) + H2O(l)
Propan-2-ol Prop-1-ene
3(a) Butan-1-ol and Butan-2-ol(position isomers of butanol) are dehydrated by conc H2SO4 at about 180oC to But-1-ene and But-2-ene respectively
Chemical equation
CH3CH2 CH2 CH2OH (l) — conc H2SO4/180oC –>CH3 CH2CH2=CH2(g) + H2O(l)
Butan-1-ol But-1-ene
CH3CHOH CH2CH3 (l)– conc H2SO4/180oC –>CH3CH=CH CH2(g) + H2O(l)
Butan-2-ol But-2-ene
(b) Butan-1-ol and Butan-2-ol are dehydrated by heating strongly aluminium (III) oxide (Al2O3) form But-1-ene and But-2-ene respectively.
Chemical equation
CH3CH2 CH2 CH2OH (l) — Heat/Al2O3 –> CH3 CH2CH2=CH2(g) + H2O(l)
Butan-1-ol But-1-ene
CH3CHOH CH2CH3 (l) — Heat/Al2O3 –> CH3CH=CH CH2(g) + H2O(l)
Butan-2-ol But-2-ene
Laboratory set up for the preparation of alkenes/ethene
Caution
(i)Ethanol is highly inflammable
(ii)Conc H2SO4 is highly corrosive on skin contact.
(iii)Common school thermometer has maximum calibration of 110oC and thus cannot be used. It breaks/cracks.
(i)Using conentrated sulphuric(VI)acid
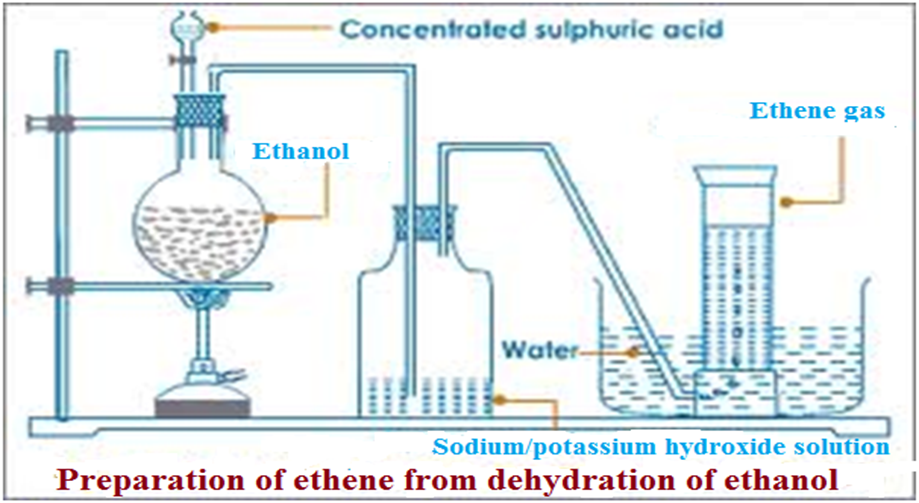
Some broken porcelain or sand should be put in the flask when heating to:
(i)prevent bumping which may break the flask.
(ii)ensure uniform and smooth boiling of the mixture
The temperatures should be maintained at above160oC.
At lower temperatures another compound –ether is predominantly formed instead of ethene gas.
(ii)Using aluminium(III)oxide
(e)Properties of alkenes
I. Physical properties
Like alkanes, alkenes are colourles gases, solids and liquids that are not poisonous.
They are slightly soluble in water.
The solubility in water decrease as the carbon chain and as the molar mass increase but very soluble in organic solvents like tetrachloromethane and methylbenzene.
The melting and boiling point increase as the carbon chain increase.
This is because of the increase in van-der-waals /intermolecular forces as the carbon chain increase.
The 1st four straight chain alkenes (ethene,propane,but-1-ene and pent-1-ene)are gases at room temperature and pressure.
The density of straight chain alkenes,like alkanes, increase with increasing carbon chain as the intermolecular forces increases reducing the volume occupied by a given mass of the alkene.
Summary of physical properties of the 1st five alkenes
| Alkene | General formula | Melting point(oC) | Boiling point(K) | State at room(298K) temperature and pressure atmosphere (101300Pa) |
| Ethene | CH2CH2 | -169 | -104 | gas |
| Propene | CH3 CHCH2 | -145 | -47 | gas |
| Butene | CH3CH2 CHCH2 | -141 | -26 | gas |
| Pent-1-ene | CH3(CH2 CHCH2 | -138 | 30 | liquid |
| Hex-1-ene | CH3(CH2) CHCH2 | -98 | 64 | liquid |
II. Chemical properties
(a)Burning/combustion
Alkenes burn with a yellow/ luminous sooty/ smoky flame in excess air to form carbon(IV) oxide and water.
Alkene + Air -> carbon(IV) oxide + water (excess air/oxygen)
Alkenes burn with a yellow/ luminous sooty/ smoky flame in limited air to form carbon(II) oxide and water.
Alkene + Air -> carbon(II) oxide + water (limited air)
Burning of alkenes with a yellow/ luminous sooty/ smoky flame is a confirmatory test for the presence of the =C=C= double bond because they have higher C:H ratio.
A homologous series with C = C double or C C triple bond is said to be unsaturated.
A homologous series with C C single bond is said to be saturated.Most of the reactions of the unsaturated compound involve trying to be saturated to form a
C C single bond .
Examples of burning alkenes
1.(a) Ethene when ignited burns with a yellow sooty flame in excess air to form carbon(IV) oxide and water.
Ethene + Air -> carbon(IV) oxide + water (excess air/oxygen)
C2H4(g) + 3O2(g) -> 2CO2(g) + 2H2O(l/g)
(b) Ethene when ignited burns with a yellow sooty flame in limited air to form carbon(II) oxide and water.
Ethene + Air -> carbon(II) oxide + water (limited air )
C2H4(g) + 3O2(g) -> 2CO2(g) + 2H2O(l/g)
2.(a) Propene when ignited burns with a yellow sooty flame in excess air to form carbon(IV) oxide and water.
Propene + Air -> carbon(IV) oxide + water (excess air/oxygen)
2C3H6(g) + 9O2(g) -> 6CO2(g) + 6H2O(l/g)
(a) Propene when ignited burns with a yellow sooty flame in limited air to form carbon(II) oxide and water.
Propene + Air -> carbon(IV) oxide + water (excess air/oxygen)
C3H6(g) + 3O2(g) -> 3CO(g) + 3H2O(l/g)
(b)Addition reactions
An addition reaction is one which an unsaturated compound reacts to form a saturated compound.Addition reactions of alkenes are named from the reagent used to cause the addtion/convert the double =C=C= to single C-C bond.
(i)Hydrogenation
Hydrogenation is an addition reaction in which hydrogen in presence of Palladium/Nickel catalyst at high temperatures react with alkenes to form alkanes.
Examples
1.When Hydrogen gas is passed through liquid vegetable and animal oil at about 180oC in presence of Nickel catalyst,solid fat is formed.
Hydrogenation is thus used to harden oils to solid fat especially margarine.
During hydrogenation, one hydrogen atom in the hydrogen molecule attach itself to one carbon and the other hydrogen to the second carbon breaking the double bond to single bond.
Chemical equation
H2C=CH2 + H2 -Ni/Pa-> H3C – CH3
H H H H
C = C + H – H – Ni/Pa -> H – C – C – H
H H H H
2.Propene undergo hydrogenation to form Propane
Chemical equation
H3C CH=CH2 + H2 -Ni/Pa-> H3C CH – CH3
H H H H H H
H C C = C + H – H – Ni/Pa-> H – C – C – C- H
H H H H H
3.Both But-1-ene and But-2-ene undergo hydrogenation to form Butane
Chemical equation
But-1-ene + Hydrogen –Ni/Pa-> Butane
H3C CH2 CH=CH2 + H2 -Ni/Pa-> H3C CH2CH – CH3
H H H H H H H H
H C C – C = C + H – H – Ni/Pa-> H – C- C – C – C- H
H H H H H H H
But-2-ene + Hydrogen –Ni/Pa-> Butane
H3C CH2 =CH CH2 + H2 -Ni/Pa-> H3C CH2CH – CH3
H H H H H H H H
H C C = C – C -H + H – H – Ni/Pa-> H – C- C – C – C- H
H H H H H H
4. But-1,3-diene should undergo hydrogenation to form Butane. The reaction uses two moles of hydrogen molecules/four hydrogen atoms to break the two double bonds.
But-1,3-diene + Hydrogen –Ni/Pa-> Butane
H2C CH CH=CH2 + 2H2 -Ni/Pa-> H3C CH2CH – CH3
H H H H H H H H
H C C – C = C -H + 2(H – H) – Ni/Pa-> H – C- C – C – C- H
H H H H
(ii) Halogenation.
Halogenation is an addition reaction in which a halogen (Fluorine, chlorine, bromine, iodine) reacts with an alkene to form an alkane.
The double bond in the alkene break and form a single bond.
The colour of the halogen fades as the number of moles of the halogens remaining unreacted decreases/reduces.
One bromine atom bond at the 1st carbon in the double bond while the other goes to the 2nd carbon.
Examples
1Ethene reacts with bromine to form 1,2-dibromoethane.
Chemical equation
H2C=CH2 + Br2 H2 Br C – CH2 Br
H H H H
C = C + Br – Br Br – C – C – Br
H H H H
Ethene + Bromine 1,2-dibromoethane
2.Propene reacts with chlorine to form 1,2-dichloropropane.
Chemical equation
H3C CH=CH2 + Cl2 H3C CHCl – CH2Cl
Propene + Chlorine 1,2-dichloropropane
H H H H H H
H C C = C + Cl – Cl H – C – C – C- Cl
H H H Cl H
H H H H H H H H
H C C – C = C + I – I H – C- C – C – C- I
H H H H H H H H
3.Both But-1-ene and But-2-ene undergo halogenation with iodine to form 1,2-diiodobutane and 2,3-diiodobutane
Chemical equation
But-1-ene + iodine 1,2 diiodobutane
H3C CH2 CH=CH2 + I2 H3C CH2CH I – CH2I
But-2-ene + Iodine 2,3-diiodobutane
H3C CH= CH-CH2 + F2 H3C CHICHI – CH3
H H H H H H H H
H C C = C – C -H + I – I H – C- C – C – C- H
H H H I I H
4. But-1,3-diene should undergo halogenation to form Butane. The reaction uses two moles of iodine molecules/four iodine atoms to break the two double bonds.
But-1,3-diene + iodine 1,2,3,4-tetraiodobutane
H2C= CH CH=CH2 + 2I2 H2CI CHICHI – CHI
H H H H H H H H
H C C – C = C -H + 2(I – I) H – C- C – C – C- H
I I I I
(iii) Reaction with hydrogen halides.
Hydrogen halides reacts with alkene to form a halogenoalkane. The double bond in the alkene break and form a single bond.
The main compound is one which the hydrogen atom bond at the carbon with more hydrogen .
Examples
1. Ethene reacts with hydrogen bromide to form bromoethane.
Chemical equation
H2C=CH2 + HBr H3 C – CH2 Br
H H H H
C = C + H – Br H – C – C – Br
H H H H
Ethene + Bromine bromoethane
2. Propene reacts with hydrogen iodide to form 2-iodopropane.
Chemical equation
H3C CH=CH2 + HI H3C CHI – CH3
| Carbon atom with more Hydrogen atoms gets extra hydrogen |
Propene + Chlorine 2-chloropropane
H H H H H H
H C C = C + H – Cl H – C – C – C– H
H H H Cl H
3. Both But-1-ene and But-2-ene reacts with hydrogen bromide to form 2- bromobutane
Chemical equation
But-1-ene + hydrogen bromide 2-bromobutane
H3C CH2 CH=CH2 + HBr H3C CH2CHBr -CH3
H H H H H H H H
H C C – C = C + H – Br H – C- C – C – C- H
H H H H H Br H
But-2-ene + Hydrogen bromide 2-bromobutane
H3C CH= CH-CH2 + HBr H3C CHBrCH2 – CH3
H H H H H H H H
H C C = C – C -H + Br – H H – C- C – C – C- H
H H H Br H H
4. But-1,3-diene react with hydrogen iodide to form 2,3- diiodobutane. The reaction uses two moles of hydrogen iodide molecules/two iodine atoms and two hydrogen atoms to break the two double bonds.
But-1,3-diene + iodine 2,3-diiodobutane
H2C= CH CH=CH2 + 2HI2 H3CCHICHI – CH3
H H H H H H H H
H C C – C = C -H + 2(H – I) H – C- C – C – C- H
H I I H
(iv) Reaction with bromine/chlorine water.
Chlorine and bromine water is formed when the halogen is dissolved in distilled water.Chlorine water has the formular HOCl(hypochlorous/chloric(I)acid) .Bromine water has the formular HOBr(hydrobromic(I)acid).
During the addition reaction .the halogen move to one carbon and the OH to the other carbon in the alkene at the =C=C= double bond to form a halogenoalkanol.
Bromine water + Alkene -> bromoalkanol
Chlorine water + Alkene -> bromoalkanol
Examples
1Ethene reacts with bromine water to form bromoethanol.
Chemical equation
H2C=CH2 + HOBr H2 Br C – CH2 OH
H H H H
C = C + Br – OH Br – C – C – OH
H H H H
Ethene + Bromine water bromoethanol
2.Propene reacts with chlorine water to form chloropropan-2-ol / 2-chloropropan-1-ol.
Chemical equation
I.H3C CH=CH2 + HOCl H3C CHCl – CH2OH
Propene + Chlorine water 2-chloropropane
H H H H H H
H C C = C + HO – Cl H – C – C – C- OH
H H H Cl H
II.H3C CH=CH2 + HOCl H3C CHOH – CH2Cl
Propene + Chlorine chloropropan-2-ol
H H H H H H
H C C = C + HO – Cl H – C – C – C- Cl
H H H OH H
3.Both But-1-ene and But-2-ene react with bromine water to form 2-bromobutan-1-ol /3-bromobutan-2-ol respectively
Chemical equation
I.But-1-ene + bromine water 2-bromobutan-1-ol
H3C CH2 CH=CH2 + HOBr H3C CH2CH Br – CH2OH
H H H H H H H H
H C C – C = C + HO– Br H – C- C – C – C- OH
H H H H H Br H
II.But-2-ene + bromine water 3-bromobutan-2-ol
H3C CH= CHCH3 + HOBr H3C CH2OHCH Br CH3
H H H H H H H H
H C C – C = C + HO– Br H – C- C – C – C- OH
H H H H H Br H
4. But-1,3-diene reacts with bromine water to form Butan-1,3-diol.
The reaction uses two moles of bromine water molecules to break the two double bonds.
But-1,3-diene + bromine water 2,4-dibromobutan-1,3-diol
H2C= CH CH=CH2 + 2HOBr H2COH CHBrCHOH CHBr
H H H H H H H H
H C C – C = C -H + 2(HO – Br) H – C- C – C – C- H
HO Br HO Br
(v) Oxidation.
Alkenes are oxidized to alkanols with duo/double functional groups by oxidizing agents.
When an alkene is bubbled into orange acidified potassium/sodium dichromate (VI) solution,the colour of the oxidizing agent changes to green.
When an alkene is bubbled into purple acidified potassium/sodium manganate(VII) solution, the oxidizing agent is decolorized.
Examples
1Ethene is oxidized to ethan-1,2-diol by acidified potassium/sodium manganate(VII) solution/ acidified potassium/sodium dichromate(VI) solution.
The purple acidified potassium/sodium manganate(VII) solution is decolorized.
The orange acidified potassium/sodium dichromate(VI) solution turns to green.
Chemical equation
H2C=CH2 [O] in H+/K2Cr2O7 HO CH2 – CH2 OH
H H H H
C = C+ [O] in H+/KMnO4 H – C – C – H
H H OH OH
Ethene + [O] in H+/KMnO4 ethan-1,2-diol
2. Propene is oxidized to propan-1,2-diol by acidified potassium/sodium manganate(VII) solution/ acidified potassium/sodium dichromate(VI) solution.
The purple acidified potassium/sodium manganate(VII) solution is decolorized.
The orange acidified potassium/sodium dichromate(VI) solution turns to green.
Chemical equation
H3C CH=CH2 [O] in H+/KMnO4 H3C CHOH – CH2OH
Propene [O] in H+/KMnO4 propan-1,2-diol
H H H H H H
H C C = C [O] in H+/KMnO4 H – C – C – C- OH
H H H OH H
3.Both But-1-ene and But-2-ene react with bromine water to form butan-1,2-diol and butan-2,3-diol
Chemical equation
I.But-1-ene + [O] in H+/KMnO4 butan-1,2-diol
H3C CH2 CH=CH2 + [O] H3C CH2CHOH – CH2OH
H H H H H H H H
H C C – C = C + [O] H – C- C – C – C- OH
H H H H H OH H
(v) Hydrolysis.
Hydrolysis is the reaction of a compound with water/addition of H-OH to a compound.
Alkenes undergo hydrolysis to form alkanols .
This takes place in two steps:
(i)Alkenes react with concentrated sulphuric(VI)acid at room temperature and pressure to form alkylhydrogen sulphate(VI).
Alkenes + concentrated sulphuric(VI)acid -> alkylhydrogen sulphate(VI)
(ii)On adding water to alkylhydrogen sulphate(VI) then warming, an alkanol is formed.
alkylhydrogen sulphate(VI) + water -warm-> Alkanol.
Examples
(i)Ethene reacts with cold concentrated sulphuric(VI)acid to form ethyl hydrogen sulphate(VII)
Chemical equation
H2C=CH2 + H2SO4 CH3 – CH2OSO3H
H H H O-SO3H
C = C + H2SO4 H – C – C – H
H H H H
Ethene + H2SO4 ethylhydrogen sulphate(VI)
(ii) Ethylhydrogen sulphate(VI) is hydrolysed by water to ethanol
Chemical equation
CH3 – CH2OSO3H + H2O CH3 – CH2OH + H2SO4
H OSO3H H OH
H – C – C – H + H2O H – C – C – H + H2SO4
H H H H
ethylhydrogen sulphate(VI) + H2O Ethanol
2. Propene reacts with cold concentrated sulphuric(VI)acid to form propyl hydrogen sulphate(VII)
Chemical equation
CH3H2C=CH2 + H2SO4 CH3CH2 – CH2OSO3H
H H H H H O-SO3H
C = C – C – H + H2SO4 H – C – C – C – H
H H H H H H
Propene + H2SO4 propylhydrogen sulphate(VI)
(ii) Propylhydrogen sulphate(VI) is hydrolysed by water to propanol
Chemical equation
CH3 – CH2OSO3H + H2O CH3 – CH2OH + H2SO4
H H OSO3H H H OH
H – C – C – C – H + H2O H – C – C – C – H + H2SO4
H H H H H H
propylhydrogen sulphate(VI) + H2O propanol
(vi) Polymerization/self addition
Addition polymerization is the process where a small unsaturated monomer (alkene ) molecule join together to form a large saturated molecule.
Only alkenes undergo addition polymerization.
Addition polymers are named from the alkene/monomer making the polymer and adding the prefix “poly” before the name of monomer to form a polyalkene
During addition polymerization
(i)the double bond in alkenes break
(ii)free radicals are formed
(iii)the free radicals collide with each other and join to form a larger molecule. The more collisions the larger the molecule.
Examples of addition polymerization
1.Formation of Polyethene
Polyethene is an addition polymer formed when ethene molecule/monomer join together to form a large molecule/polymer at high temperatures and pressure.
During polymerization:
(i)many molecules are brought nearer to each other by the high pressure(which reduces the volume occupied by reacting particles)
H H H H H H H H
C = C + C = C + C = C + C = C + …
H H H H H H H H
Ethene + Ethene + Ethene + Ethene + …
(ii)the double bond joining the ethane molecule break to free readicals
H H H H H H H H
•C – C• + •C – C• + •C – C• + •C – C• + …
H H H H H H H H
Ethene radical + Ethene radical + Ethene radical + Ethene radical + …
(iii)the free radicals collide with each other and join to form a larger molecule
H H H H H H H H lone pair of electrons
•C – C – C – C – C – C – C – C• + …
H H H H H H H H
Lone pair of electrons can be used to join more monomers to form longer polyethene.
Polyethene molecule can be represented as:
H H H H H H H H extension of
molecule/polymer
– C – C – C – C – C – C – C – C-
H H H H H H H H
Since the molecule is a repetition of one monomer, then the polymer is:
H H
( C – C )n
H H
Where n is the number of monomers in the polymer. The number of monomers in the polymer can be determined from the molar mass of the polymer and monomer from the relationship:
Number of monomers/repeating units in monomer = Molar mass polymer
Molar mass monomer
Examples
Polythene has a molar mass of 4760.Calculate the number of ethene molecules in the polymer(C=12.0, H=1.0 )
Number of monomers/repeating units in polyomer = Molar mass polymer
Molar mass monomer
=> Molar mass ethene (C2H4 )= 28 Molar mass polyethene = 4760
Substituting 4760 = 170 ethene molecules
28
The commercial name of polyethene is polythene.
It is an elastic, tough, transparent and durable plastic.
Polythene is used:
(i)in making plastic bag
(ii)bowls and plastic bags
(iii)packaging materials
2.Formation of Polychlorethene
Polychloroethene is an addition polymer formed when chloroethene molecule/monomer join together to form a large molecule/polymer at high temperatures and pressure.
During polymerization:
(i)many molecules are brought nearer to each other by the high pressure(which reduces the volume occupied by reacting particles)
H H H H H H H H
C = C + C = C + C = C + C = C + …
H Cl H Cl H Cl H Cl
chloroethene + chloroethene + chloroethene + chloroethene + …
(ii)the double bond joining the chloroethene molecule break to free radicals
H H H H H H H H
•C – C• + •C – C• + •C – C• + •C – C• + …
H Cl H Cl H Cl H Cl
(iii)the free radicals collide with each other and join to form a larger molecule
H H H H H H H H lone pair of electrons
•C – C – C – C – C – C – C – C• + …
H Cl H Cl H Cl H Cl
Lone pair of electrons can be used to join more monomers to form longer polychloroethene.
Polychloroethene molecule can be represented as:
H H H H H H H H extension of
molecule/polymer
– C – C – C – C – C – C – C – C- + …
H Cl H Cl H Cl H Cl
Since the molecule is a repetition of one monomer, then the polymer is:
H H
( C – C )n
H Cl
Examples
Polychlorothene has a molar mass of 4760.Calculate the number of chlorethene molecules in the polymer(C=12.0, H=1.0,Cl=35.5 )
Number of monomers/repeating units in monomer = Molar mass polymer
Molar mass monomer
=> Molar mass ethene (C2H3Cl )= 62.5 Molar mass polyethene = 4760
Substituting 4760 = 77.16 => 77 polychloroethene molecules(whole number)
62.5
The commercial name of polychloroethene is polyvinylchloride(PVC). It is a tough, non-transparent and durable plastic. PVC is used:
(i)in making plastic rope
(ii)water pipes
(iii)crates and boxes
3.Formation of Polyphenylethene
Polyphenylethene is an addition polymer formed when phenylethene molecule/monomer join together to form a large molecule/polymer at high temperatures and pressure.
During polymerization:
(i)many molecules are brought nearer to each other by the high pressure(which reduces the volume occupied by reacting particles)
H H H H H H H H
C = C + C = C + C = C + C = C + …
H C6H5 H C6H5 H C6H5 H C6H5
phenylethene + phenylethene + phenylethene + phenylethene + …
(ii)the double bond joining the phenylethene molecule break to free radicals
H H H H H H H H
•C – C• + •C – C• + •C – C• + •C – C• + …
H C6H5 H C6H5 H C6H5 H C6H5
(iii)the free radicals collide with each other and join to form a larger molecule
H H H H H H H H lone pair of electrons
• C – C – C – C – C – C – C – C • + …
H C6H5 H C6H5 H C6H5 H C6H5
Lone pair of electrons can be used to join more monomers to form longer polyphenylethene.
Polyphenylethene molecule can be represented as:
H H H H H H H H
– C – C – C – C – C – C – C – C –
H C6H5 H C6H5 H C6H5 H C6H5
Since the molecule is a repetition of one monomer, then the polymer is:
H H
( C – C )n
H C6H5
Examples
Polyphenylthene has a molar mass of 4760.Calculate the number of phenylethene molecules in the polymer(C=12.0, H=1.0, )
Number of monomers/repeating units in monomer = Molar mass polymer
Molar mass monomer
=> Molar mass ethene (C8H8 )= 104 Molar mass polyethene = 4760
Substituting 4760 = 45.7692 =>45 polyphenylethene molecules(whole number)
104
The commercial name of polyphenylethene is polystyrene. It is a very light durable plastic. Polystyrene is used:
(i)in making packaging material for carrying delicate items like computers, radion,calculators.
(ii)ceiling tiles
(iii)clothe linings
4.Formation of Polypropene
Polypropene is an addition polymer formed when propene molecule/monomer join together to form a large molecule/polymer at high temperatures and pressure.
During polymerization:
(i)many molecules are brought nearer to each other by the high pressure(which reduces the volume occupied by reacting particles)
H H H H H H H H
C = C + C = C + C = C + C = C + …
H CH3 H CH3 H CH3 H CH3
propene + propene + propene + propene + …
(ii)the double bond joining the phenylethene molecule break to free radicals
H H H H H H H H
•C – C• + •C – C• + •C – C• + •C – C• + …
H CH3 H CH3 H CH3 H CH3
(iii)the free radicals collide with each other and join to form a larger molecule
H H H H H H H H lone pair of electrons
• C – C – C – C – C – C – C – C • + …
H CH3 H CH3 H CH3 H CH3
Lone pair of electrons can be used to join more monomers to form longer propene.
propene molecule can be represented as:
H H H H H H H H
– C – C – C – C – C – C – C – C –
H CH3 H CH3 H CH3 H CH3
Since the molecule is a repetition of one monomer, then the polymer is:
H H
( C – C )n
H CH3
Examples
Polypropene has a molar mass of 4760.Calculate the number of propene molecules in the polymer(C=12.0, H=1.0, )
Number of monomers/repeating units in monomer = Molar mass polymer
Molar mass monomer
=> Molar mass propene (C3H8 )= 44 Molar mass polyethene = 4760
Substituting 4760 = 108.1818 =>108 propene molecules(whole number)
44
The commercial name of polyphenylethene is polystyrene. It is a very light durable plastic. Polystyrene is used:
(i)in making packaging material for carrying delicate items like computers, radion,calculators.
(ii)ceiling tiles
(iii)clothe linings
5.Formation of Polytetrafluorothene
Polytetrafluorothene is an addition polymer formed when tetrafluoroethene molecule/monomer join together to form a large molecule/polymer at high temperatures and pressure.
During polymerization:
(i)many molecules are brought nearer to each other by the high pressure(which reduces the volume occupied by reacting particles)
F F F F F F F F
C = C + C = C + C = C + C = C + …
F F F F F F F F
tetrafluoroethene + tetrafluoroethene+ tetrafluoroethene+ tetrafluoroethene + …
(ii)the double bond joining the tetrafluoroethene molecule break to free radicals
F F F F F F F F
•C – C• + •C – C• + •C – C• + •C – C• + …
F F F F F F F F
(iii)the free radicals collide with each other and join to form a larger molecule
F F F F F F F F lone pair of electrons
•C – C – C – C – C – C – C – C• + …
F F F F F F F F
Lone pair of electrons can be used to join more monomers to form longer polytetrafluoroethene.
polytetrafluoroethene molecule can be represented as:
F F F F F F F F extension of
molecule/polymer
– C – C – C – C – C – C – C – C- + …
F F F F F F F F
Since the molecule is a repetition of one monomer, then the polymer is:
F F
( C – C )n
F F
Examples
Polytetrafluorothene has a molar mass of 4760.Calculate the number of tetrafluoroethene molecules in the polymer(C=12.0, ,F=19 )
Number of monomers/repeating units in monomer = Molar mass polymer
Molar mass monomer
=> Molar mass ethene (C2F4 )= 62.5 Molar mass polyethene = 4760
Substituting 4760 = 77.16 => 77 polychloroethene molecules(whole number)
62.5
The commercial name of polytetrafluorethene(P.T.F.E) is Teflon(P.T.F.E). It is a tough, non-transparent and durable plastic. PVC is used:
(i)in making plastic rope
(ii)water pipes
(iii)crates and boxes
6.Formation of rubber from Latex
Natural rubber is obtained from rubber trees.
During harvesting an incision is made on the rubber tree to produce a milky white substance called latex.
Latex is a mixture of rubber and lots of water.
The latex is then added an acid to coagulate the rubber.
Natural rubber is a polymer of 2-methylbut-1,3-diene ;
During natural polymerization to rubber, one double C=C bond break to self add to another molecule. The double bond remaining move to carbon “2” thus;
H CH3 H H H CH3 H H
– C – C = C – C – C – C = C – C –
H H H H
Generally the structure of rubber is thus;
H CH3 H H
-(- C – C = C – C -)n–
H H
Pure rubber is soft and sticky.It is used to make erasers, car tyres. Most of it is vulcanized.Vulcanization is the process of heating rubber with sulphur to make it harder/tougher.
During vulcanization the sulphur atoms form a cross link between chains of rubber molecules/polymers. This decreases the number of C=C double bonds in the polymer.
| Sulphur atoms make cross link between polymers |
H CH3 H H H CH3 H H
– C – C – C – C – C – C – C – C –
H S H H S H
H CH3 S H H CH3 S H
– C – C – C – C – C – C – C – C –
H H H H H H
Vulcanized rubber is used to make tyres, shoes and valves.
7.Formation of synthetic rubber
Synthetic rubber is able to resist action of oil,abrasion and organic solvents which rubber cannot.
Common synthetic rubber is a polymer of 2-chlorobut-1,3-diene ;
H Cl H H
CH2=C (Cl CH = CH2 H – C = C – C = C – H
During polymerization to synthetic rubber, one double C=C bond is broken to self add to another molecule. The double bond remaining move to carbon “2” thus;
H Cl H H H Cl H H
– C – C = C – C – C – C = C – C –
H H H H
Generally the structure of rubber is thus;
H Cl H H
-(- C – C = C – C -)n–
H H
Rubber is thus strengthened through vulcanization and manufacture of synthetic rubber.
(c)Test for the presence of – C = C – double bond.
(i)Burning/combustion
All unsaturated hydrocarbons with a – C = C – or – C = C – bond burn with a yellow sooty flame.
Experiment
Scoop a sample of the substance provided in a clean metallic spatula. Introduce it on a Bunsen burner.
| Observation | Inference |
| Solid melt then burns with a yellow sooty flame | – C = C –, – C = C – bond |
(ii)Oxidation by acidified KMnO4/K2Cr2O7
Bromine water ,Chlorine water and Oxidizing agentsacidified KMnO4/K2Cr2O7change to unique colour in presence of – C = C –
or – C = C – bond.
Experiment
Scoop a sample of the substance provided into a clean test tube. Add 10cm3 of distilled water. Shake. Take a portion of the solution mixture. Add three drops of acidified KMnO4/K2Cr2O7 .
| Observation | Inference |
| Acidified KMnO4 decolorized Orange colour of acidified K2Cr2O7turns green Bromine water is decolorized Chlorine water is decolorized | – C = C – – C = C – bond |
(d)Some uses of Alkenes
1. In the manufacture of plastic
2. Hydrolysis of ethene is used in industrial manufacture of ethanol.
3. In ripening of fruits.
4. In the manufacture of detergents.
