A factory uses 63.0 kg of 68% pure nitric (V) acid per day to produce an ammonium fertilizer for an agricultural county. If the density of the acid is 1.42 gcm-3, calculate:
(i) the concentration of the acid used in moles per litre.
Molar mass HNO3 = 63
Method 1
Moles of HNO3 in 1cm3 = Mass in 1cm3 1.42 => 1.42 = 0.0225 moles
Molar mass HNO3 63
Molarity = Moles x 1000=>0.0225 moles x10000 = 22.5molesdm-3/M
1 cm3
100% = 22.5molesdm-3/M
68% = 68 x 22.5 = 15.3M/ molesdm-3
100
Method 2
Moles of HNO3 in 1000cm3 = Mass in 1000cm3 =>1.42 x1000
Molar mass HNO3 63
=22.5397 molesdm-3/M
100% = 22.5397 molesdm-3/M
68% = 68 x 22.5397 = 15.327 molesdm-3
100
(ii) the volume of ammonia gas at r.t.p used. (H=1.0, N=14.0, O=16.0, one mole of gas = 24 dm-3 at r.t.p)
Chemical equation
HNO3 (as) + NH3 (g) -> NH4NO3 (as)
Mole ratio HNO3 (as): NH3 (g) = 1: 1
1 mole HNO3 (as) -> 24dm3 NH3 (g)
15.327 mole HNO3 (as) ->15.327 mole x 24 dm3 = 367.848dm3
1dm3
(iii) the number of crops which can be applied the fertilizer if each crop requires 4.0g.
HNO3 (aq) + NH3 (g) -> NH4NO3 (aq)
Molar mass NH4NO3 =80 g
Mole ratio HNO3: NH4NO3 = 1: 1
Mass of HNO3 in 63.0 kg = 68% x 63 =42.84kg
1 mole HNO3 (aq) =63g -> 80g NH4NO3
(42.84×1000) gHNO3 (aq) -> (42.84×1000) g x 80
63
= 54400g
Mass of fertilizer = 54400g = 13600 crops
Mass per crop 4.0
E. NITRATE (V) NO3– and NITRATE (III) NO2– Salts
Nitrate (V) /NO3– and Nitrate (III) /NO2– are salts derived from Nitric (V)/HNO3 and Nitric (III)/HNO2 acids. Both HNO3 and HNO2 are monobasic acids with only one ignitable hydrogen in a molecule.
Only KNO2, NaNO2 and NH4NO2 exist. All metallic nitrate (V) salts exist.
All Nitrate (V) /NO3– and Nitrate (III) /NO2– are soluble/dissolve in water.
(a)Effect of heat on Nitrate (V) /NO3– and Nitrate (III) /NO2– salts (Test for presence of Nitrate (V) /NO3– ions in solid state)
1. All Nitrate (III) /NO2– salts are not affected by gentle or strong heating except ammonium nitrate (III) NH4NO2.
Ammonium nitrate (III) NH4NO2 is a colourless solid that decompose to form Nitrogen gas and water.
Chemical equation
NH4NO2 (s) -> H2O (l) + N2 (g)
This reaction is used to prepare small amounts of Nitrogen in a school laboratory.
2. All Nitrate (V) /NO3– salts decompose on strong heating:
Experiment
Put ½ spatula full of sodium nitrate (V) into a test tube. Place moist blue/red litmus papers on the mouth of the test tube. Heat strongly when test tube is slanted.
Test the gases produced using glowing splint
Caution (i) Wear safety gas mask and hand gloves
(ii)Lead (II) nitrate (V) decomposes to Lead (II) oxide that reacts and fuses with the test tube permanently.
Repeat with potassium nitrate(V), copper(II) nitrate(V), Lead(II)nitrate(V), silver nitrate(V), Zinc nitrate(V), Magnesium nitrate(V) and Ammonium nitrate(V).
Observations
Cracking sound
Brown fumes/gas produced except in potassium nitrate (V) and Sodium nitrate (V)
Glowing splint relights/rekindles but feebly in Ammonium nitrate(V).
Black solid residue with copper(II) nitrate(V)
White residue/solid with sodium nitrate(V), potassium nitrate(V),silver nitrate(V), Magnesium nitrate(V)
Yellow residue/solid when hot but white on cooling with Zinc nitrate(V)
Brown residue/solid when hot but yellow on cooling with Lead(II)nitrate(V)
Explanation
1. Potassium nitrate(V) and Sodium nitrate(V) decomposes on strong heating to form potassium nitrate(III) and Sodium nitrate(III) producing Oxygen gas. Oxygen gas relights/rekindles a glowing splint.
Chemical equation.
2KNO3(s) -> 2KNO2(s) + O2 (g)
2NaNO3(s) -> 2NaNO2(s) + O2 (g)
2.Heavy metal nitrate(V)salts decomposes to form the oxide, brown nitrogen (IV) oxide and Oxygen gas.
Copper(II)oxide is black.Zinc oxide is yellow when hot and white when cool/cold. Lead(II)oxide is yellow when cold/cool and brown when hot/heated.
Hydrated copper(II)nitrate is blue. On heating it melts and dissolves in its water of crystallization to form a green solution. When all the water of crystallization has evaporated,the nitrate(V)salt decomposes to black Copper(II)oxide and a mixture of brown nitrogen(IV)oxide gas and colourless Oxygen gas.
Chemical equation
2Cu(NO3)2(s) -> 2CuO (s) + 4NO2(g) + O2(s)
2Ca(NO3)2(s) -> 2CaO (s) + 4NO2(g) + O2(s)
2Zn(NO3)2(s) -> 2ZnO (s) + 4NO2(g) + O2(s)
2Mg(NO3)2(s) -> 2MgO (s) + 4NO2(g) + O2(s)
2Pb(NO3)2(s) -> 2PbO (s) + 4NO2(g) + O2(s)
2Fe(NO3)2(s) -> 2FeO (s) + 4NO2(g) + O2(s)
Silver nitrate(V)and Mercury(II)nitrate decomposes to the corresponding metal and a mixture of brown nitrogen(IV)oxide gas and colourless Oxygen gas.
Chemical equation
2AgNO3 (s) -> 2Ag (s) + 2NO2(g) + O2(s)
Hg(NO3)2(s) -> Hg(l) + 2NO2(g) + O2(s)
The production/evolution of brown fumes of Nitrogen(IV)oxide gas on heating a salt is a confirmatory test for presence of NO3– ions of heavy metals
(b)Brown ring test (Test for presence of Nitrate(V) /NO3– ions in aqueous/ solution state)
Experiment
Place 5cm3 of Potassium nitrate(V)solution onto a clean test tube. Add 8 drops of freshly prepared Iron(II)sulphate(VI)solution. Swirl/ shake.
Using a test tube holder to firmly slant and hold the test tube, carefully add 5cm3 of Concentrated sulphuric (VI) acid down along the side of test tube.Do not shake the test tube contents.
Caution: Concentrated sulphuric (VI) acid is highly corrosive.
Observation.
Both Potassium nitrate(V)and freshly prepared Iron(II)sulphate (VI)do not form layers
On adding Concentrated Sulphuric(VI)acid,two layers are formed.
A brown ring is formed between the layers.
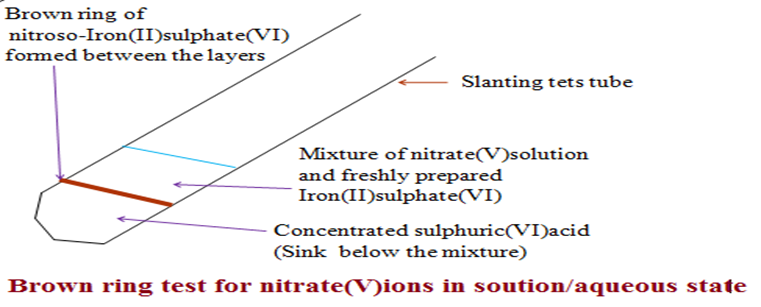
Explanation
All nitrate(V)salts are soluble. They form a miscible mixture when added freshly prepared Iron(II)sulphate(VI)solution. Concentrated sulphuric(VI)acid is denser than the miscible mixture thus settle at the bottom.
At the junction of the layers, the acid reacts with nitrate(V)salts to form Nitric(V)acid/HNO3. Nitric(V)acid/HNO3 is reduced to Nitrogen (II)oxide by the Iron(II)sulphate(VI) salt to form the complex compound Nitroso-iron(II)sulphate(VI)/FeSO4.NO . Nitroso-iron(II)sulphate(VI) is brown in colour.It forms a thin layer at the junction between concentrated sulphuric (VI)acid and the miscible mixture of freshly prepared Iron(II) sulphate(VI) and the nitrate(V)salts as a brown ring.
Chemical equation
FeSO4(aq) + NO(g) -> FeSO4.NO(aq)
(Nitroso-iron(II)sulphate(VI)complex)
The brown ring disappear if shaken because concentrated sulphuric (VI)acid mixes with the aqueous solution generating a lot of heat which decomposes Nitroso-iron(II)sulphate(VI)/FeSO4.NO to iron(II)sulphate(VI) and Nitrogen(II)oxide.
Chemical equation
FeSO4.NO(aq) ->FeSO4(aq) + NO(g) ->
Iron(II)sulphate(VI) solution is easily/readily oxidized to iron(III)sulphate(VI) on exposure to air/oxygen. The brown ring test thus require freshly prepared Iron(II) sulphate(VI) solution
(c)Devardas alloy test (Test for presence of Nitrate(V) /NO3– ions in aqueous/ solution state)
Experiment
Place 5cm3 of Potassium nitrate(V)solution onto a clean test tube. Add 5 drops of sodium hydroxide solution. Swirl/ shake. Add a piece of aluminium foil to the mixture.Heat.Test any gases produced using both blue and red litmus papers.
Observation. Inference
Effervescence/bubbles/fizzing
colourless gas that has a pungent smell of urine NO3–
Blue limus paper remain blue
Red litmus paper turn red.
Explanation
The Devardas alloy test for NO3– ions in solution was developed by the Italian scientist Artulo Devarda(1859-1944)
When a NO3–salt is added sodium hydroxide and aluminium foil, effervescence of ammonia gas is a confirmatory test for NO3– ions.
