1.Electrolysis is defined simply as the decomposition of a compound by an electric current/electricity.
A compound that is decomposed by an electric current is called an electrolyte. Some electrolytes are weak while others are strong.
2.Strong electrolytes are those that are fully ionized/dissociated into (many) ions. Common strong electrolytes include:
(i)all mineral acids
(ii)all strong alkalis/sodium hydroxide/potassium hydroxide.
(iii)all soluble salts
3.Weak electrolytes are those that are partially/partly ionized/dissociated into (few) ions.
Common weak electrolytes include:
(i)all organic acids
(ii)all bases except sodium hydroxide/potassium hydroxide.
(iii)Water
4. A compound that is not decomposed by an electric current is called non-electrolyte. Non-electrolytes are those compounds /substances that exist as molecules and thus cannot ionize/dissociate into(any) ions .
Common non-electrolytes include:
(i) most organic solvents (e.g. petrol/paraffin/benzene/methylbenzene/ethanol)
(ii)all hydrocarbons(alkanes /alkenes/alkynes)
(iii)Chemicals of life(e.g. proteins, carbohydrates, lipids, starch, sugar)
5. An electrolytes in solid state have fused /joined ions and therefore do not conduct electricity but the ions (cations and anions) are free and mobile in molten and aqueous (solution, dissolved in water) state.
6.During electrolysis, the free ions are attracted to the electrodes. An electrode is a rod through which current enter and leave the electrolyte during electrolysis. An electrode that does not influence/alter the products of electrolysis is called an inert electrode.
Common inert electrodes include:
(i)Platinum
(ii)Carbon graphite
Platinum is not usually used in a school laboratory because it is very expensive. Carbon graphite is easily/readily and cheaply available (from used dry cells).
7.The positive electrode is called Anode.The anode is the electrode through which current enter the electrolyte/electrons leave the electrolyte
8.The negative electrode is called Cathode. The cathode is the electrode through which current leave the electrolyte / electrons enter the electrolyte
9. During the electrolysis, free anions are attracted to the anode where they lose /donate electrons to form neutral atoms/molecules. i.e.
M(l) -> M+(l) + e (for cations from molten electrolytes)
M(s) -> M+(aq) + e (for cations from electrolytes in aqueous state / solution / dissolved in water)
The neutral atoms /molecules form the products of electrolysis at the anode. This is called discharge at anode
10. During electrolysis, free cations are attracted to the cathode where they gain /accept/acquire electrons to form neutral atoms/molecules.
X+ (aq) + 2e -> X(s) (for cations from electrolytes in aqueous state / solution / dissolved in water)
2X+ (l) + 2e -> X (l) (for cations from molten electrolytes)
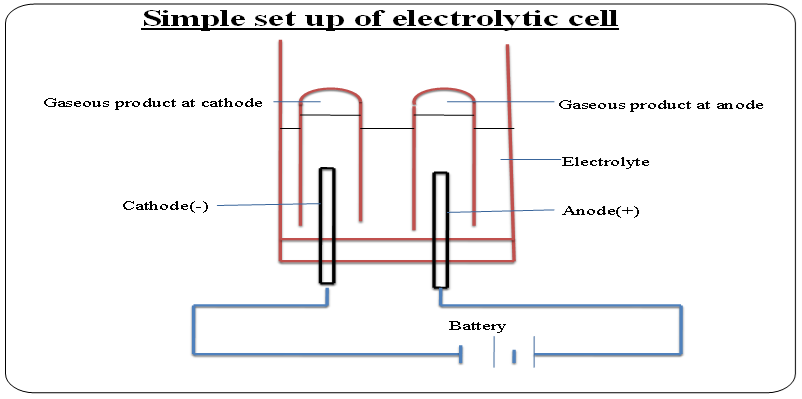
The neutral atoms /molecules form the products of electrolysis at the cathode. This is called discharge at cathode.11. The below set up shows an electrolytic cell.

12. For a compound /salt containing only two ion/binary salt the products of electrolysis in an electrolytic cell can be determined as in the below examples:
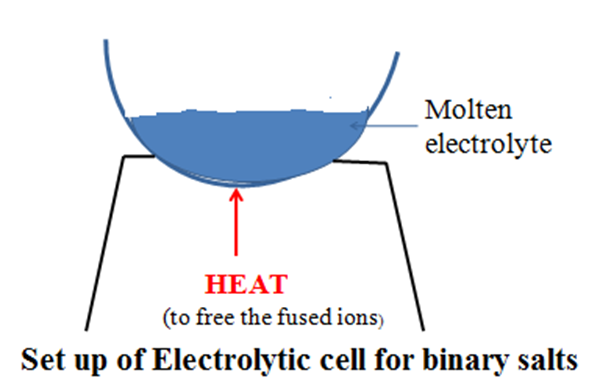
a)To determine the products of electrolysis of molten Lead(II)chloride
(i)Decomposition of electrolyte into free ions;
PbCl2 (l) -> Pb 2+(l) + 2Cl–(l)
(Compound decomposed into free cation and anion in liquid state)
(ii)At the cathode/negative electrode(-);
Pb 2+(l) + 2e -> Pb (l)
(Cation / Pb 2+ gains / accepts / acquires electrons to form free atom)
(iii)At the anode/positive electrode(+);
2Cl–(l) -> Cl2 (g) + 2e
(Anion / Cl– donate/lose electrons to form free atom then agas molecule)
(iv)Products of electrolysis therefore are;
I.At the cathode grey beads /solid lead metal.
II.At the anode pale green chlorine gas.
b)To determine the products of electrolysis of molten Zinc bromide
(i)Decomposition of electrolyte into free ions;
ZnBr2 (l) -> Zn 2+(l) + 2Br–(l)
(Compound decomposed into free cation and anion in liquid state)
(ii)At the cathode/negative electrode(-);
Zn 2+(l) + 2e -> Zn(l)
(Cation / Zn2+ gains / accepts / acquires electrons to form free atom)
(iii)At the anode/positive electrode(+);
2Br–(l) -> Br2 (g) + 2e
(Anion / Br– donate/lose electrons to form free atom then aliquid molecule whichchange to gas on heating)
(iv)Products of electrolysis therefore are;
I.At the cathode grey beads /solid Zinc metal.
II.At the anode red bromine liquid / red/brown bromine gas.
c)To determine the products of electrolysis of molten sodium chloride
(i)Decomposition of electrolyte into free ions;
NaCl (l) -> Na +(l) + Cl–(l)
(Compound decomposed into free cation and anion in liquid state)
(ii)At the cathode/negative electrode(-);
2Na+(l) + 2e -> Na (l)
(Cation / Na+ gains / accepts / acquires electrons to form free atom)
(iii)At the anode/positive electrode(+);
2Cl–(l) -> Cl2 (g) + 2e
(Anion / Cl– donate/lose electrons to form free atom then agas molecule)
(iv)Products of electrolysis therefore are;
I.At the cathode grey beads /solid sodium metal.
II.At the anode pale green chlorine gas.
d)To determine the products of electrolysis of molten Aluminium (III)oxide
(i)Decomposition of electrolyte into free ions;
Al2O3 (l) -> 2Al 3+(l) + 3O2-(l)
(Compound decomposed into free cation and anion in liquid state)
(ii)At the cathode/negative electrode(-);
4Al 3+ (l) + 12e -> 4Al (l)
(Cation / Al 3+ gains / accepts / acquires electrons to form free atom)
(iii)At the anode/positive electrode(+);
6O2-(l) -> 3O2 (g) + 12e
(Anion /6O2- donate/lose 12 electrons to form free atom then threegas molecule)
(iv)Products of electrolysis therefore are;
I.At the cathode grey beads /solid aluminium metal.
II.At the anode colourless gas that relights/rekindles glowing splint.
13. For a compound /salt mixture containing many ions in an electrolytic cell, the discharge of ions in the cell depend on the following factors:
- Position of cations and anions in the electrochemical series
1. Most electropositive cations require more energy to reduce (gain electrons) and thus not readily discharged. The higher elements /metals in the electrochemical series the less easily/readily it is discharged at the cathode in the electrolytic cell.
Table I showing the relative ease of discharge of cations in an electrolytic cell
K+(aq) + e -> K(s) (least readily/easily discharged)
Na+(aq) + e -> Na(s)
Ca2+(aq) + 2e -> Ca(s)
Mg2+(aq) + 2e -> Mg(s)
Al3+(aq) + 3e -> Al(s)
Zn2+(aq) + 2e -> Zn(s)
Fe2+(aq) + 2e -> Fe(s)
Pb2+(aq) + 2e -> Pb(s)
2H+(aq) + 2e -> H2(g) (hydrogen is usually “metallic”)
Cu2+(aq) + 2e -> Cu(s)
Hg2+(aq) + 2e -> Hg(s)
Ag+(aq) + e -> Ag(s) (most readily/easily discharged)
2.The OH– ion is the most readily/easily discharged anion . All the other anionic radicals(SO42- ,SO32- ,CO32- ,HSO4– ,HCO3– ,NO3– ,PO43-)are not/never discharged. The ease of discharge of halogen ions increase down the group.
Table II showing the relative ease of discharge of anions in an electrolytic cell
4OH– (aq) -> 2H2O(l) + O2 (g) + 4e (most readily/easily discharged)
2 I–(aq) -> I2(aq) + 2e
2 Br–(aq) -> Br2(aq) + 2e
2 Cl–(aq) -> Cl2(aq) + 2e
2 F–(aq) -> F2(aq) + 2e
SO42- ,SO32- ,CO32- ,HSO4– ,HCO3– ,NO3– ,PO43- not/never/rarely discharged.
3.(a)When two or more cations are attracted to the cathode, the ion lower in the electrochemical series is discharged instead of that which is higher as per the table I above. This is called selective/preferential discharge at cathode.
(b)When two or more anions are attracted to the anode, the ion higher in the electrochemical series is discharged instead of that which is lower as per the table I above. This is called selective/preferential discharge at anode.
4.The following experiments shows the influence /effect of selective/preferential discharge on the products of electrolysis:
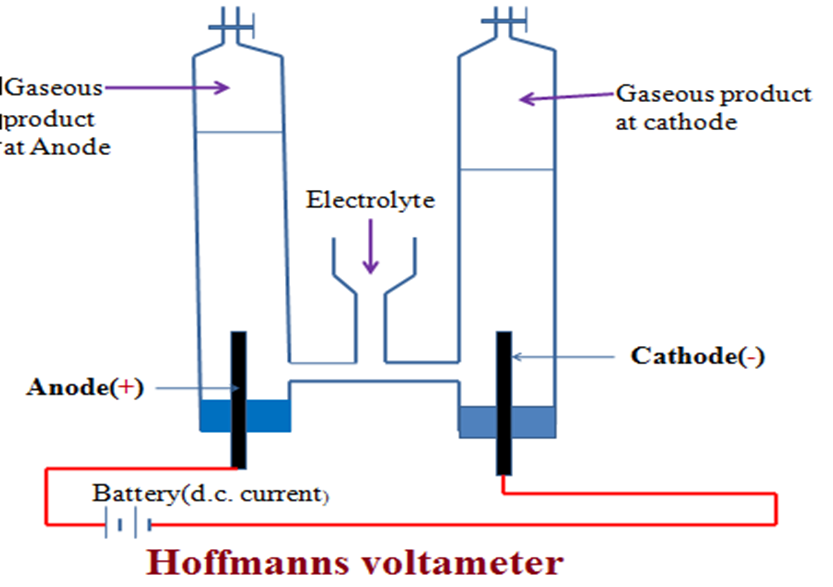
(i)Electrolysis of acidified water/dilute sulphuric(VI) acid
Fill the Hoffmann voltameter with dilute sulphuric(VI) acid. Connect the Hoffmann voltameter to a d.c. electric supply. Note the observations at each electrode.
Electrolytic cell set up during electrolysis of acidified water/dilute sulphuric(VI) acid