Radioactivity is the spontaneous disintegration/decay of an unstable nuclide.
A nuclide is an atom with defined mass number (number of protons and neutrons), atomic number and definite energy.
Radioactivity takes place in the nucleus of an atom unlike chemical reactions that take place in the energy levels involving electrons.
A nuclide is said to be stable if its neutron: proton ratio is equal to one (n/p = 1)
All nuclide therefore try to attain n/p = 1 by undergoing radioactivity.
Examples
(i)Oxygen nuclide with 168 O has 8 neutrons and 8 protons in the nucleus therefore an n/p = 1 thus stable and do not decay/disintegrate.
(ii)Chlorine nuclide with 3517 Cl has 18 neutrons and 17 protons in the nucleus therefore an n/p = 1.0588 thus unstable and decays/disintegrates to try to attain n/p = 1.
(ii)Uranium nuclide with 23792 U has 206 neutrons and 92 protons in the nucleus therefore an n/p = 2.2391 thus more unstable than 23592 U and thus more readily decays / disintegrates to try to attain n/p = 1.
(iii) Chlorine nuclide with 3717 Cl has 20 neutrons and 17 protons in the nucleus therefore an n/p = 1.1765 thus more unstable than 3517 Cl and thus more readily decays / disintegrates to try to attain n/p = 1.
(iv)Uranium nuclide with 23592 U has 143 neutrons and 92 protons in the nucleus therefore an n/p = 1.5543 thus more stable than 237 92U but also readily decays / disintegrates to try to attain n/p = 1.
All unstable nuclides naturally try to attain nuclear stability with the production of:
(i)alpha(α) particle decay
The alpha (α) particle has the following main characteristic:
i)is positively charged(like protons)
ii) has mass number 4 and atomic number 2 therefore equal to a charged Helium atom ( 42He2+)
iii) have very low penetrating power and thus can be stopped /blocked/shielded by a thin sheet of paper.
iv) have high ionizing power thus cause a lot of damage to living cells.
v) a nuclide undergoing α-decay has its mass number reduced by 4 and its atomic number reduced by 2
Examples of alpha decay
210 84 Pb -> x 82 Pb + 42He 2+
210 84 Pb -> 206 82 Pb + 42He 2+
226 88 Ra -> 222 y Rn + 42He 2+
226 88 Ra -> 222 86 Rn + 42He 2+
x y U -> 23490 Th + 42He 2+
238 92 U -> 23490 Th + 42He 2+
x y U -> 23088 Ra + 2 42He 2+
238 92 U -> 23088 Ra + 2 42He 2+
210 84 U -> xy W + 10 α
210 84 U -> 17064 W + 10 α
210 92U -> xy W + 6 α
210 92U -> 18680W + 6 α
(ii)Beta (β) particle decay
The Beta (β) particle has the following main characteristic:
i)is negatively charged(like electrons)
ii)has no mass number and atomic number negative one(-1) therefore equal to a fast moving electron (0 -1e)
iii) have medium penetrating power and thus can be stopped /blocked/shielded by a thin sheet of aluminium foil.
iv) have medium ionizing power thus cause less damage to living cells than the α particle.
v) a nuclide undergoing β -decay has its mass number remain the same and its atomic number increase by 1
Examples of beta (β) decay
1.23 x Na -> 2312Mg + 0 -1e
23 11 Na -> 2312Mg + 0 -1e
2. 234 x Th -> y91 Pa + 0 -1e
234 90 Th -> y91 Pa + 0 -1e
3. 20770Y -> x y Pb + 30 -1e
20770Y -> 207 73Pb + 30 -1e
4.x yC -> 147N + 0 -1e
14 6C -> 147N + 0 -1e
5. 1 xn -> y1H + 0 -1e
1 0n -> 11H + 0 -1e
6. 42He -> 411H + x 0 -1e
42He -> 411H + 2 0 -1e
7. 22888Ra -> 22890Th + x β
22888Ra -> 22892Th + 4 β
8. 23290Th -> 21282Pb + 2 β + x α
23290Th -> 21282Pb + 2 β + 5 α
9. 23892U -> 22688 Ra + x β + 3 α
23892U -> 22688 Ra + 2 β + 3 α
10. 21884Po -> 20682Pb + x β + 3 α
21884Po -> 20682Pb + 4β + 3 α
(iii)Gamma (y) particle decay
The gamma (y) particle has the following main characteristic:
i)is neither negatively charged(like electrons/beta) nor positively charged(like protons/alpha) therefore neutral.
ii)has no mass number and atomic number therefore equal to electromagnetic waves.
iii) have very high penetrating power and thus can be stopped /blocked/shielded by a thick block of lead..
iv) have very low ionizing power thus cause less damage to living cells unless on prolonged exposure..
v) a nuclide undergoing y -decay has its mass numberand its atomic number remain the same.
Examples of gamma (y) decay
- 3717Cl -> 3717Cl + y
- 146C -> 146C + y
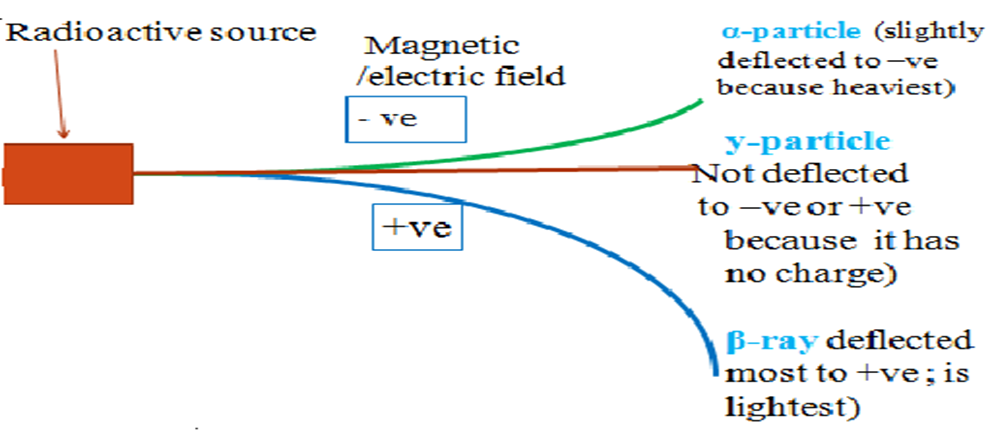
The sketch diagram below shows the penetrating power of the radiations from a radioactive nuclide.
radioactive nuclide sheet of paper aluminium foil thick block of lead
(radiation source) (block α-rays) (block β-rays) block y-rays)
α-rays β-rays y-rays
The sketch diagram below illustrates the effect of electric /magnetic field on the three radiations from a radioactive nuclide
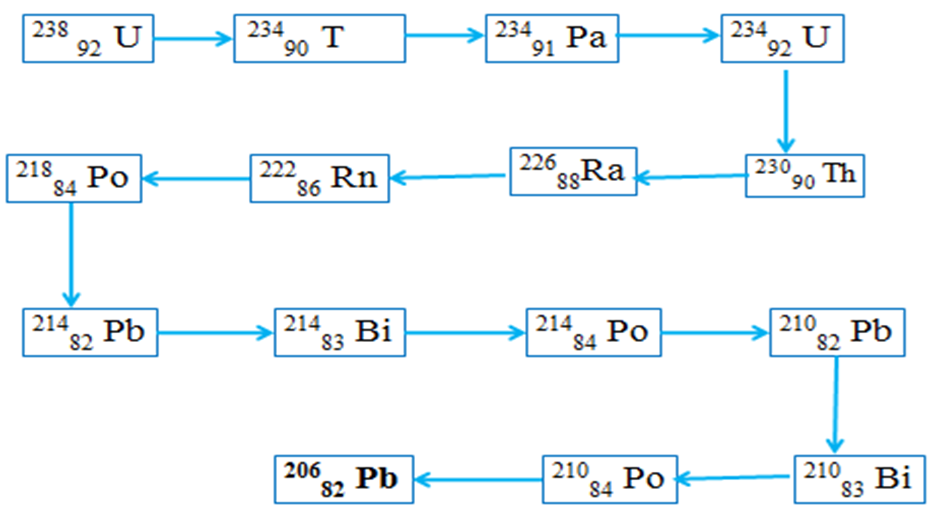
Radioactive disintegration/decay naturally produces the stable 20682Pb nuclide /isotope of lead.Below is the 238 92 U natural decay series. Identify the particle emitted in each case
Write the nuclear equation for the disintegration from :
(i)238 92 U to 23490 T
238 92 U -> 23490 T + 4 2 He 2+
238 92 U -> 23490 T + α
(ii)238 92 U to 222 84 Rn
238 92 U -> 22284 Rn + 4 4 2 He 2+
238 92 U -> 22284 Rn + 4α
230 90 Th undergoes alpha decay to222 86 Rn. Find the number ofα particles emitted. Write the nuclear equation for the disintegration.
Working
230 90 Th -> 222 86 Rn + x 4 2 He
Method 1
Using mass numbers
230 = 222 + 4 x => 4 x = 230 – 222 = 8
x = 8 / 4 = 2 α
Using atomic numbers
90 = 86 + 2 x => 2 x = 90 – 86 = 4
x = 4 / 2 = 2 α
Nuclear equation
230 90 Th -> 222 86 Rn + 2 4 2 He
214 82 Pb undergoes beta decay to214 84 Rn. Find the number ofβ particles emitted. Write the nuclear equation for the disintegration.
Working
214 82 Pb -> 214 84 Rn + x 0 -1 e
Using atomic numbers only
82 = 84 – x => -x = 82 – 84 = -2
x = 2 β
Nuclear equation
214 82 Pb -> 214 84 Rn + 2 0 -1 e
238 92 U undergoes beta and alpha decay to206 82 Pb. Find the number ofβ andαparticles emitted. Write the nuclear equation for the disintegration.
Working
238 92 U -> 206 82 Pb + x 0 -1 e + y 4 2 He
Using Mass numbers only
238 = 206 + 4y => 4y = 238 – 206 = 32
y = 32 = 8 α
4
Using atomic numbers only and substituting the 8 α(above)
238 92 U -> 206 82 Pb + 8 4 2 He + x 0 -1 e
92 = 82 + 16 + – x
=> 92 – (82 + 16) = – x
x = 6 β
Nuclear equation
238 92 U -> 206 82 Pb + 6 0 -1 e + 8 4 2 He
298 92 U undergoes alpha and beta decay to214 83 Bi. Find the number of α and β particles emitted. Write the nuclear equation for the disintegration.
Working
298 92 U -> 210 83 Bi + x 4 2 He + y 0 -1 e
Using Mass numbers only
298 = 214 + 4x => 4x = 298 – 214 = 84
y = 84 = 21 α
4
Using atomic numbers only and substituting the 21 α (above)
238 92 U -> 214 83Bi + 21 4 2 He + y 0 -1 e
92 = 83 + 42 + – y
=> 92 – (83 + 42) = – x
x = 33 β
Nuclear equation
298 92 U -> 210 83 Bi + 21 4 2 He + 33 0 -1 e
