Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
H2 SO4(aq) -> SO42-(aq) + 2H+(aq)
II. Name the ions in acidified water that are attracted/move to:
Cathode- H+(aq) from either sulphuric(VI) acid(H2 SO4) or water(H2O)
Anode– SO42-(aq) from sulphuric (VI) acid(H2 SO4) and OH– (aq) from water(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode 4H+(aq) + 4e -> 2H2(g)
Anode 4OH– (aq) -> 2H2O(l) + O2 (g) + 4e
(4OH– ions selectively discharged instead of SO42- ions at the anode)
IV. Name the products of electrolysis of acidified water.
Cathode-Hydrogen gas(colourless gas that extinguishes burning splint with explosion/ “pop” sound
Anode-Oxygen gas(colourless gas that relights /rekindles glowing splint)
V. Explain the difference in volume of products at the cathode and anode.
The four(4) electrons donated/lost by OH– ions to form 1 molecule/1volume/1mole of oxygen (O2)gas at the anode are gained/acquired/accepted by the four H+(aq) ionsto form 2 molecule/2volume/2mole of Hydrogen (H2)gas at the cathode.
The volume of Oxygen gas at the anode is thus a half the volume of Hydrogen produced at the cathode/ The volume of Hydrogen gas at the cathode is thus a twice the volume of Oxygen produced at the anode.
VI. Why is electrolysis of dilute sulphuric(VI) acid called “electrolysis of (acidified) water”?
The ratio of H2 (g): O2 (g) is 2:1 as they are combined in water. This implies/means that water in the electrolyte is being decomposed into hydrogen and Oxygen gases. The electrolysis of dilute sulphuric acid is therefore called “electrolysis of acidified water.”
VI. Explain the changes in concentration of the electrolyte during electrolysis of acidified water”
The concentration of dilute sulphuric (VI) acid increases. Water in the electrolyte is decomposed into Hydrogen and Oxygen gases that escape. The concentration /mole of acid present in a given volume of solution thus continue increasing/rising.
(ii)Electrolysis of Magnesium sulphate(VI) solution
Fill the Hoffmann voltameter with dilute sulphuric(VI) acid. Connect the Hoffmann voltameter to a d.c. electric supply. Note the observations at each electrode.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
Mg SO4(aq) -> SO42-(aq) + Mg2+(aq)
II. Name the ions in Magnesium sulphate(VI) solution that are attracted/move to:
Cathode- Mg2+(aq) fromMagnesium sulphate(VI) solution (Mg SO4) and H+(aq) fromwater(H2O)
Anode– SO42-(aq) fromMagnesium sulphate(VI) solution (Mg SO4) and OH– (aq) from water(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode 4H+(aq) + 4e -> 2H2(g)
H+ ions selectively discharged instead of Mg2+ ions at the cathode)
Anode 4OH– (aq) -> 2H2O(l) + O2 (g) + 4e
(4OH– ions selectively discharged instead of SO42- ions at the anode)
IV. Name the products of electrolysis of Magnesium sulphate(VI) solution
Cathode-Hydrogen gas(colourless gas that extinguishes burning splint with explosion/ “pop” sound
Anode-Oxygen gas(colourless gas that relights /rekindles glowing splint)
V. Explain the difference in volume of products at the cathode and anode.
The four(4) electrons donated/lost by OH– ions to form 1 molecule/1volume/1mole of oxygen (O2)gas at the anode are gained/acquired/accepted by the four H+(aq) ionsto form 2 molecule/2volume/2mole of Hydrogen (H2)gas at the cathode.
The volume of Oxygen gas at the anode is thus a half the volume of Hydrogen produced at the cathode/ The volume of Hydrogen gas at the cathode is thus a twice the volume of Oxygen produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of Magnesium sulphate(VI) solution
The concentration of dilute Magnesium sulphate(VI) solution increases.
The ratio of H2 (g): O2 (g) is 2:1 as they are combined in water.
Water in the electrolyte is decomposed into Hydrogen and Oxygen gases that escape as products.
The concentration /mole of acid present in a given volume of Magnesium sulphate(VI) solution thus continue increasing/rising.
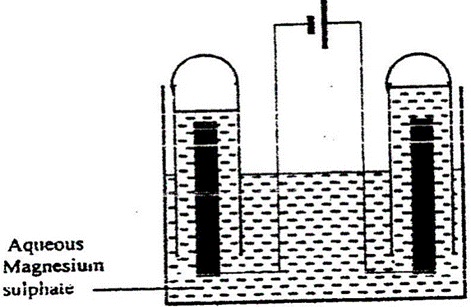
The set – up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes.
The volume of Oxygen gas at the anod e is thus a half the volume of Hydrogen produced at the cathode/ The volume of Hydrogen gas at the cathode is thus a twice the volume of Oxygen produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of Magnesium sulphate(VI) solution
The concentration of dilute Magnesium sulphate(VI) solution increases.
The ratio of H2 (g): O2 (g) is 2:1 as they are combined in water.
Water in the electrolyte is decomposed into Hydrogen and Oxygen gases that escape as products.
The concentration /mole of acid present in a given volume of Magnesium sulphate(VI) solution thus continue increasing/rising.
The set – up below was used during the electrolysis of aqueous magnesium sulphate using inert electrodes.
Name a suitable pair of electrodes for this experiment
Identify the ions and cations in the solution
On the diagram label the cathode
Write ionic equations for the reactions that took place at the anode.
Explain the change that occurred to the concentration of magnesium sulphate solution during the experience.
During the electrolysis a current of 2 amperes was passed through the solution for 4 hours. Calculate the volume of the gas produced at the anode.(1 faraday 96500 coulombs and volume of a gas at room temperature is 24000cm3)
One of the uses of electrolysis is electroplating
What is meant by electroplating?
Give tow reasons why electroplating is necessary.
- Concentration of the electrolytes
1.High concentrations of cations and/or anions at the electrodes block the ion/s that is likely to be discharged at the electrode. This is called over voltage. A concentrated solution therefore produces different products of electrolysis from a dilute one.
2. The following experiments show the influence/effect of concentration of electrolyte on the products of electrolysis.
(i)Electrolysis of dilute and concentrated(brine)sodium chloride solution
I. Dissolve about 0.5 g of pure sodium chloride crystals in 100cm3 of water. Place the solution in an electrolytic cell. Note the observations at each electrode for 10 minutes. Transfer the set up into a fume chamber/open and continue to make observations for a further 10 minute.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
NaCl(aq) -> Cl–(aq) + Na+(aq)
II. Name the ions in sodium chloride solution that are attracted/move to:
Cathode- Na+(aq) fromSodium chloride solution (NaCl) and H+(aq) fromwater(H2O)
Anode– Cl–(aq) fromsodiumchloride solution (NaCl) and OH– (aq) from water(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode 4H+(aq) + 4e -> 2H2(g)
H+ ions selectively discharged instead of Na+ ions at the cathode)
Anode 4OH– (aq) -> 2H2O(l) + O2 (g) + 4e
(4OH– ions selectively discharged instead of Cl– ions at the anode)
IV. Name the products of electrolysis of dilute sodium chloride solution
Cathode-Hydrogen gas(colourless gas that extinguishes burning splint with explosion/ “pop” sound
Anode-Oxygen gas(colourless gas that relights /rekindles glowing splint)
V. Explain the difference in volume of products at the cathode and anode.
Four(4) electrons donated/lost by OH– ions to form 1 molecule/1volume/1mole of oxygen (O2)gas at the anode are gained/acquired/accepted by four H+(aq) ionsto form 2 molecule/2volume/2mole of Hydrogen (H2)gas at the cathode.
The volume of Oxygen gas at the anode is half the volume of Hydrogen produced at the cathode/ The volume of Hydrogen gas at the cathode is twice the volume of Oxygen produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of sodium chloride solution
The concentration of dilute sodium chloride solution increases.
The ratio of H2 (g): O2 (g) is 2:1 as they are combined in water. Water in the electrolyte is decomposed into Hydrogen and Oxygen gases that escape as products. The concentration /moles of salt present in a given volume of sodium chloride solution continue increasing/rising.
II. Dissolve about 20 g of pure sodium chloride crystals in 100cm3 of water. Place the solution in an electrolytic cell. Note the observations continuously at each electrode for 30 minutes in a fume chamber/open.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
NaCl(aq) -> Cl–(aq) + Na+(aq)
II. Name the ions in sodium chloride solution that are attracted/move to:
Cathode- Na+(aq) fromSodium chloride solution (NaCl) and H+(aq) fromwater(H2O)
Anode– Cl–(aq) fromsodium chloride solution (NaCl) and OH– (aq) from water(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode 2H+(aq) + 2e -> H2(g)
H+ ions selectively discharged instead of Na+ ions at the cathode)
Anode 2Cl– (aq) -> Cl2(g) + 4e
(Cl– ions with a higher concentration block the discharge of OH– ions at the anode)
IV. Name the products of electrolysis of concentrated sodium chloride solution/brine
Cathode-Hydrogen gas(colourless gas that extinguishes burning splint with explosion/ “pop” sound
Anode-Chlorine gas(pale green gas that bleaches damp/moist/wet litmus papers)
V. Explain the difference in volume of products at the cathode and anode.
Two (2) electrons donated/lost by Cl– ions to form 1 molecule/1volume/1mole of Chlorine (Cl2)gas at the anode are gained/acquired/accepted by two H+(aq) ionsto form 1 molecule/1volume/1mole of Hydrogen (H2)gas at the cathode.
The volume of Chlorine gas at the anode is equal to the volume of Hydrogen produced at the cathode/ The volume of Hydrogen gas at the cathode is equal to the volume of Chlorine produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of concentrated sodium chloride solution/brine
The concentration of concentrated sodium chloride solution/brine increases.
The ratio of Cl2 (g): H2 (g) is 1:1 as they are combined in water.
Water in the electrolyte is decomposed into only Hydrogen gas that escapes as products at cathode.
The concentration /moles of OH– (aq) and Na+ ion (as NaOH) present in a given volume of electrolyte continue increasing/rising.
This makes the electrolyte strongly alkaline with high pH.
As the electrolysis of brine continues the concentration of Cl– ions decrease and oxygen gas start being liberated at anode.
The electrolyte pH is thus lowered and the concentration of brine starts again increasing.
(ii)Electrolysis of dilute and concentrated Hydrochloric acid solution
I. Prepare about 50cm3 of 0.05 M of dilute Hydrochloric acid in 100cm3 solution. Place the solution in an electrolytic cell. Note the observations at each electrode for 10 minutes.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
HCl(aq) -> Cl–(aq) + H+(aq)
II. Name the ions in dilute Hydrochloric acid solution that are attracted/move to:
Cathode- H+(aq) fromdilute Hydrochloric acid (HCl) and H+(aq) fromwater(H2O)
Anode– Cl–(aq) fromdilute Hydrochloric acid (HCl) and OH– (aq) from water(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode 4H+(aq) + 4e -> 2H2(g)
H+ ions selectively discharged instead of Na+ ions at the cathode)
Anode 4OH– (aq) -> H2O(l) +O2+ 4e
(4OH– ions selectively discharged instead of Cl– ions at the anode)
IV. Name the products of electrolysis of dilute Hydrochloric acid
Cathode-Hydrogen gas(colourless gas that extinguishes burning splint with explosion/ “pop” sound
Anode-Oxygen gas(colourless gas that relights /rekindles glowing splint)
V. Explain the difference in volume of products at the cathode and anode.
Four(4) electrons donated/lost by OH– ions to form 1 molecule/1volume/1mole of oxygen (O2)gas at the anode are gained/acquired/accepted by four H+(aq) ionsto form 2 molecule/2volume/2mole of Hydrogen (H2)gas at the cathode.
The volume of Oxygen gas at the anode is half the volume of Hydrogen produced at the cathode/ The volume of Hydrogen gas at the cathode is twice the volume of Oxygen produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of dilute Hydrochloric acid
The concentration of dilute Hydrochloric acid increases.
The ratio of H2 (g): O2 (g) is 2:1 as they are combined in water. Water in the electrolyte is decomposed into Hydrogen and Oxygen gases that escape as products. The concentration /moles of HCl present in a given volume of dilute Hydrochloric acid continue increasing/rising.
II. Prepare about 50cm3 of 2M of Hydrochloric acid in 100cm3 solution. Place the solution in an electrolytic cell. Note the observations at each electrode for 30 minutes
CautionThis experiment should be done in the open/fume chamber.
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
HCl(aq) -> Cl–(aq) + H+(aq)
II. Name the ions in 2M Hydrochloric acid solution that are attracted/move to:
Cathode- H+(aq) fromdilute Hydrochloric acid (HCl) and H+(aq) fromwater(H2O)
Anode– Cl–(aq) fromdilute Hydrochloric acid (HCl) and OH– (aq) from water(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode 4H+(aq) + 4e -> 2H2(g)
H+ ions selectively discharged instead of Na+ ions at the cathode)
Anode 2Cl– (aq) -> Cl2+ 2e
(OH– ions concentration is low.Cl– ions concentration is higher at the anode thus cause over voltage/block discharge of OH– ions)
IV. Name the products of electrolysis of 2M Hydrochloric acid
Cathode-Hydrogen gas(colourless gas that extinguishes burning splint with explosion/ “pop” sound
Anode-Chlorine gas(Pale green gas that bleaches blue/red moist/wet/damp litmus papers)
V. Explain the difference in volume of products at the cathode and anode.
Two(2) electrons donated/lost by Cl– ions to form 1 molecule/1volume/1mole of Chlorine (Cl2)gas at the anode are gained/acquired/accepted by two H+(aq) ionsto form 1 molecule/1volume/1mole of Hydrogen (H2)gas at the cathode.
The volume of Chlorine gas at the anode is equal to the volume of Hydrogen produced at the cathode/ The volume of Hydrogen gas at the cathode is twice the volume of Chlorine produced at the anode.
VI. Explain the changes in concentration of the electrolyte during electrolysis of 2M Hydrochloric acid
The concentration of Hydrochloric acid decreases.
The ratio of H2 (g): Cl2 (g) is 1:1 as they are combined in Hydrochloric acid.
Water in the electrolyte is decomposed only into Hydrogen gas that escapes as products at the cathode.
There is a net accumulation of excess OH– (aq) ions in solution.
This makes the electrolyte strongly alkaline with high pH.
- Nature of electrodes used in the electrolytic cell
Inert electrodes (carbon-graphite and platinum) do not alter the expected products of electrolysis in an electrolytic cell. If another/different electrode is used in the electrolytic cell it alters/influences/changes the expected products of electrolysis.
The examples below illustrate the influence of the nature of electrode on the products of electrolysis:
(i)Electrolysis of copper(II) sulphate(VI) solution
I. Using carbon-graphite electrodes
Weigh Carbon -graphite electrodes. Record the masses of the electrodes in table I below. Place the electrodes in 1Mcopper(II) sulphate(VI) solution in a beaker. Set up an electrolytic cell.
Close the switch and pass current for about 20 minutes. Observe each electrode and any changes in electrolyte. Remove the electrodes from the electrolyte. Wash with acetone/propanone and allow them to dry. Reweigh each electrode.
Sample results
| Mass of cathode before electrolysis | 23.4 g | Mass of anode before electrolysis | 22.4 g |
| Mass of cathode after electrolysis | 25.4 g | Mass of anode after electrolysis | 22.4 g |
| Brown solid deposit at the cathode after electrolysis | – | Bubbles of colourless gas that relight splint | – |
| Blue colour of electrolyte fades/become less blue | – | Blue colour of electrolyte fades /become less blue | – |
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
CuSO4(aq) -> SO42-(aq) + Cu2+(aq)
II. Name the ions in 1M copper(II) sulphate(VI) solution that are attracted/move to:
Cathode- Cu2+ (aq) fromcopper(II) sulphate(VI) solution and H+(aq) fromwater(H2O)
Anode– SO42-(aq) fromcopper(II) sulphate(VI) solution and OH– (aq) fromwater(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode 2Cu2+ (aq) + 4e -> 2Cu(g)
Cu2+ ions are lower than H+ ions in the electrochemical series therefore selectively discharged at the cathode.)
Anode 4OH– (aq) -> H2O(l) + O2+ 4e
(OH– ions ions are higher than SO42- ions in the electrochemical series therefore selectively discharged at the cathode.))
IV. Name the products of electrolysis of 1M copper(II) sulphate(VI) solution
Cathode-2 moles of copper metal asbrown solid coat
Anode–Oxygen gas(Colourless gas that relights /rekindles glowing splint)
V. Explain the changes that take place at the cathode and anode.
Four(4) electrons donated/lost by OH– ions to form 1 molecule/1volume/1mole of Oxygen (O2)gas at the anode are gained/acquired/accepted by two Cu2+(aq) ionsto form 2 moles of brown copper solid that deposit itself at the cathode.
The moles of oxygen gas at the anode is equal to the moles of copper produced at the cathode
VI. Explain the changes in electrolyte during electrolysis of 1M copper (II) sulphate(VI) solution.
(i)The pH of copper(II) sulphate(VI) solution lowers/decreases. The salt becomes more acidic. Water in the electrolyte is decomposed only into Oxygen gas (from the OH– ions) that escapes as products at the anode. There is a net accumulation of excess H+ (aq) ions in solution. This makes the electrolyte strongly acidic with low pH.
(ii) Cu2+ (aq) ions are responsible for the blue colour of the electrolyte/ copper(II) sulphate (VI) solution. As electrolysis continues, blue Cu2+ (aq) ions gain electrons to form brown Copper. The blue colour of electrolyte therefore fades/become less blue.
(iii)Copper is deposited at the cathode. This increases the mass of the cathode.OH– ions that produce Oxygen gas at anode come from water. Oxygen escapes out/away without increasing the mass of anode.
II. Using copper electrodes
Weigh clean copper plates electrodes. Record the masses of the electrodes in table I below. Place the electrodes in 1Mcopper(II) sulphate(VI) solution in a beaker. Set up an electrolytic cell.
Close the switch and pass current for about 20 minutes. Observe each electrode and any changes in electrolyte. Remove the electrodes from the electrolyte. Wash with acetone/propanone and allow them to dry. Reweigh each electrode.
Sample results
| Mass of cathode before electrolysis | 23.4 g | Mass of anode before electrolysis | 22.4 g |
| Mass of cathode after electrolysis | 25.4 g | Mass of anode after electrolysis | 20.4 g |
| Brown solid deposit at the cathode after electrolysis | – | Anode decrease insize/erodes/wear off | – |
| Blue colour of electrolyte remain blue | – | Blue colour of electrolyte remain blue | – |
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
CuSO4(aq) -> SO42-(aq) + Cu2+(aq)
II. Name the ions in 1M copper(II) sulphate(VI) solution that are attracted/move to:
Cathode- Cu2+ (aq) fromcopper(II) sulphate(VI) solution and H+(aq) fromwater(H2O)
Anode– SO42-(aq) fromcopper(II) sulphate(VI) solution and OH– (aq) fromwater(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode Cu2+ (aq) + 2e -> Cu(s)
Cu2+ ions are lower than H+ ions in the electrochemical series therefore selectively discharged at the cathode.)
Anode Cu (s) -> Cu2+(aq) + 2e
(Both OH– ions and SO42- ions move to the anode but none is discharged. The copper anode itself ionizes/dissolves/dissociate because less energy is used to remove an electron/ionize /dissociate copper atoms than OH– ions.
IV. Name the products of electrolysis of 1M copper(II) sulphate(VI) solution using copper electrodes.
Cathode-1 moles of copper metal asbrown solid coat(Cathode increase/deposits)
Anode-Anode erodes/decrease in size
V. Explain the changes that take place during the electrolytic process
(i)Cathode
-Cu2+ ionsare lower than H+ ions in the electrochemical series therefore selectively discharged at the cathode. Cu2+ ions have greater tendency to accept/gain/acquire electrons to form brown copper atoms/solid that deposit itself and increase the mass/size of the cathode.The copper deposited at the cathode is pure
-H+ ions accumulate around the cathode. Electrolyte thus becomes strongly acidic around the cathode.
-Cu2+ ions in solution are responsiblefor the blue colour of electrolyte. Blue colour of electrolyte fade around the cathode.
(ii)Anode
Copper atom at the anode easily ionizes to release electrons. The anode therefore keeps decreasing in mass/eroding. The amount of copper that dissolve/erode is equal to the mass of copper deposited. This is called electrode ionization.
Electrode ionization is where the anode erodes/decrease and the cathode deposits/increase during electrolysis. The overall concentration of the electrolyte remains constant
14.In industries electrolysis has the following uses/applications:
(a)Extraction of reactive metals from their ores.
Potassium, sodium ,magnesium, and aluminium are extracted from their ores using electrolytic methods.
(b)Purifying copper after exraction from copper pyrites ores.
Copper obtained from copper pyrites ores is not pure. After extraction, the copper is refined by electrolysing copper(II)sulphate(VI) solution using the impure copper as anode and a thin strip of pure copper as cathode. Electrode ionization take place there:
(i)At the cathode; Cu2+ (aq) + 2e -> Cu(s) (Pure copper deposits on the strip
(ii)At the anode; Cu(s) ->Cu2+ (aq) + 2e (impure copper erodes/dissolves)
(c)Electroplating
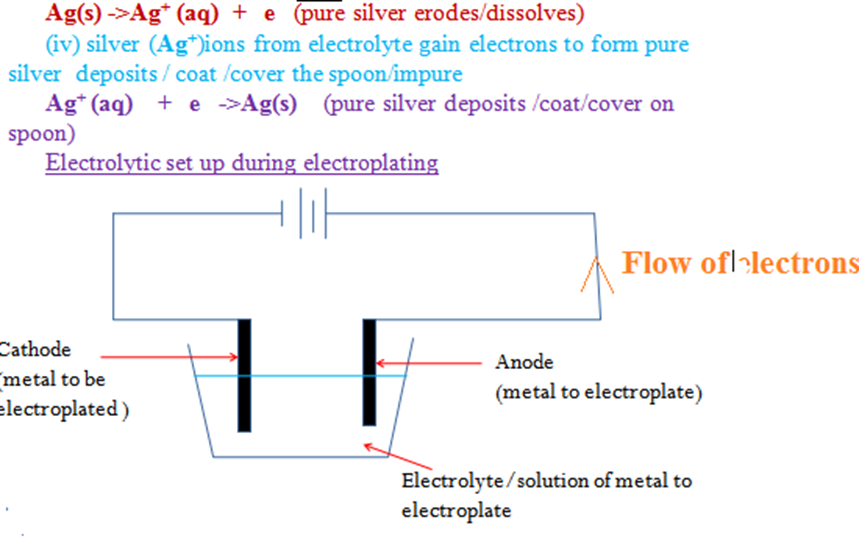
The label EPNS(Electro Plated Nickel Silver) on some steel/metallic utensils mean they are plated/coated with silver and/or Nickel to improve their appearance(add their aesthetic value)and prevent/slow corrosion(rusting of iron). Electroplating is the process of coating a metal with another metal using an electric current. During electroplating,the cathode is made of the metal to be coated/impure.
Example:
During the electroplating of a spoon with silver
(i)the spoon/impure is placed as the cathode(negative terminal of battery)
(ii)the pure silver is placed as the anode(positive terminal of battery)
(iii)the pure silver erodes/ionizes/dissociates to release electrons:
Ag(s) ->Ag+ (aq) + e (impure silver erodes/dissolves)
(iv) silver (Ag+)ions from electrolyte gain electrons to form pure silver deposits / coat /cover the spoon/impure
Ag+ (aq) + e ->Ag(s) (pure silver deposits /coat/cover on spoon)
15.The quantitative amount of products of electrolysis can be determined by applying Faradays 1st law of electrolysis.
Faradays 1st law of electrolysis states that “the mass/amount of substance liberated/produced/used during electrolysis is directly proportional to the quantity of of electricity passed/used.”
(a)The SI unit of quantity of electricity is the coulomb(C). The coulomb may be defined as the quantity of electricity passed/used when a current of one ampere flow for one second.i.e;
1Coulomb = 1 Ampere x 1Second
The Ampere is the SI unit of current(I)
The Second is the SI unit of time(t) therefore;
Quantity of electricity(in Coulombs) = Current(I) x time(t)
Practice examples
1. A current of 2 amperes was passed through an electrolytic cell for 20 minutes. Calculate the quantity of electric charge produced.
Working:
Quantity of electricity(in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 2 x (20 x 60)
= 2400 C
2. A current of 2 amperes was passed through an electrolytic.96500 coulombs of charge were produced. Calculate the time taken.
Working:
Time(t) in seconds = Quantity of electricity(in Coulombs)
Current(I) in amperes
Substituting = 96500
2
= 48250 seconds
3. 96500 coulombs of charge were produced after 10 minutes in an electrolytic cell . Calculate the amount of current used.
Working:
Current(I) in amperes = Quantity of electricity(in Coulombs) Time(t) in seconds
Substituting/converting time to second= 96500
10 x 60
= 160.8333 Amperes
(b)The quantity of electricity required for one mole of electrons at the anode/cathode is called the Faraday constant(F). It is about 96500 Coulombs.i.e
The number of Faradays used /required is equal to the number of electrons used at cathode/anode during the electrolytic process. e.g.
Cu2+ require to gain 2 moles of electrons=2 Faradays =2 x 96500 coulombs of electricity at the cathode.
Al3+ require to gain 3 moles of electrons=3 Faradays =3 x 96500 coulombs of electricity at the cathode
Na+ require to gain 1 moles of electrons=1 Faradays =1 x 96500 coulombs of electricity at the cathode
2H+ require to gain 2 moles of electrons=2 Faradays =2 x 96500 coulombs of electricity at the cathode to form 1molecule of hydrogen gas
2O2– require to lose/donate 4 moles of electrons=4 Faradays =4 x 96500 coulombs of electricity at the anode to form 1molecule of Oxygen O2 gas.
4OH– require to lose/donate 4 moles of electrons=4 Faradays =4 x 96500 coulombs of electricity at the anode to form 1molecule of Oxygen gas and 2 molecules of water.
(c)The mass/amount of products at the cathode/anode is related to the molar mass of the substance and/or the volume of gases at standard/room temperature and pressure as in the below examples:
Practice examples
1.Calculate the mass of copper deposited at the cathode when a steady current of 4.0 amperes is passed through copper(II)sulphate(VI) for 30 minutes in an electrolytic cell. (Cu=63.5, 1F = 96500C)
Working:
Quantity of electricity(in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 4 x (30 x 60)
= 7200 C
Equation at the cathode: Cu2+ (aq) + 2e -> Cu(s)
2 mole of electrons = 2 Faradays = 2 x 96500 C produce a mass =molar mass of copper thus;
2 x 96500C -> 63.5 g
72000C -> 7200 x 63.5 = 2.3689 g of copper
2 x 96500
2.a)If 3.2 g of Lead were deposited when a current of 2.5 amperes was passed through an electrolytic cell of molten Lead(II)bromide for 20 minutes, determine the Faraday constant.(Pb = 207)
Working:
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 2.5 x (20 x 60)
= 3000 C
If 3.2g of Lead -> 3000C
Then 207 g of Lead -> 207 x 3000 = 194062.5 C
3.2
Equation at the cathode: Pb2+ (l) + 2e -> Pb(l)
From the equation: 2 moles of electrons = 2 Faradays = 194062.5 C
1mole of electrons = 1 Faraday => 194062.5 = 97031.25 C
2
b)What is the volume of bromine vapour produced at the anode at room temperature(1mole of gas at room temperature and pressure = 24000cm3)
Method 1
Equation at the anode: Br– (l) -> Br2(g) + 2e
From the equation: 2 moles of electrons = 2 Faradays = 194062.5 C -> 24000cm3
3000 C -> 3000 x 24000
194062.5
=371.0145cm3
Method 2
Equation at the anode: Br– (l) -> Br2(g) + 2e
Mole ratio of products at Cathode: anode = 1:1
Moles of Lead at cathode = 3.2 = 0.0155moles = moles of Bromine
207
1 moles of bromine vapour -> 24000cm3
0.0155moles of Bromine -> 0.0155 x 24000 = 372 cm3
Method 3
Equation at the anode: Br– (l) -> Br2(g) + 2e
Ratio of Faradays used to form products at Cathode: anode = 2:2
=> 2 x 97031.25 C produce 24000cm3 of bromine vapour
Then: 3000 C -> 3000 x 24000cm3 = 371.0145cm3
2 x 97031.25
3.What mass of copper remain from 2.0 at the anode if a solution of copper(II)sulphate(VI) is electrolysed using a current of 1 ampere flowing through an electrolytic cell for 20 minutes.(Cu= 63.5, 1Faraday = 96487 coulombs)
Working:
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 1 x (20 x 60)
= 1200 C
Equation at the cathode: Cu2+ (aq) + 2e -> Cu(s)
2 mole of electrons = 2 Faradays = 2 x 96500 C erode/dissolve a mass =molar mass of copper thus;
2 x 96500C -> 63.5 g
1200C -> 1200 x 63.5 = 0.3948g of copper deposited
2 x 96500
Mass of copper remaining = Original mass – mass dissolved/eroded
=> 2.0 -0.3948 = 1.6052 g of copper remain
4. Calculate the current passed if a mass of 0.234 g of copper is deposited in 4 minutes during electrolysis of a solution of copper (II)sulphate(VI).
(Cu= 63.5 ,1F = 96500C)
Working:
Equation at the cathode: Cu(s) -> Cu2+ (aq) + 2e
2 mole of electrons = 2 Faradays = 2 x 96500 C produce a mass =molar mass of copper thus;
63.5 g -> 2 x 96500C
0.234 g -> 0.234 x 2 x 96500 = 711.2126 C
63.5
Current(I) in amperes = Quantity of electricity(in Coulombs) Time(t) in seconds
Substituting/converting time to second= 711.2126 C
4x 60
= 2.9634 Amperes
5. (a)What quantity of electricity will deposit a mass of 2.43 g of Zinc during electrolysis of a solution of Zinc (II)sulphate(VI).
(Zn= 65 ,1F = 96500C)
Working:
Equation at the cathode: Zn2+ (aq) + 2e -> Zn(s)
2 mole of electrons = 2 Faradays = 2 x 96500 C erode/dissolve a mass =molar mass of Zinc thus;
65 g -> 2 x 96500
2.43 g -> 2.43 x 2 x 96500 = 7215.2308 C
65
(b)Calculate the time (in minutes) it would take during electrolysis of the solution of Zinc (II)sulphate(VI) above if a current of 4.0 Amperes is used.
Time(t) in seconds = Quantity of electricity(in Coulombs)
Current(I) in amperes
Substituting = 7215.2308 = 1803.8077 seconds = 30.0635 minutes
4 60
6.When a current of 1.5 amperes was passed through a cell containing M3+ ions of metal M for 15 minutes, the mass at cathode increased by 0.26 g.(Faraday constant = 96500C
a) Calculate the quantity of electricity used.
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 1.5 x (15 x 60)
= 1350 C
- Determine the relative atomic mass of metal M
Equation at the cathode: M3+ (aq) + 3e -> M(s)
1350 C of electricity -> 0.26 g of metal M
3 mole of electrons = 3 Faradays = 3 x 96500 C produce a mass =molar mass of M thus;
RAM of M = 0.26 g x 3 x 96500 = 55.7556(No units)
1350
7.An element “P” has a relative atomic mass 88.When a current of 0.5 amperes was passed through fused chloride of “P” for 32 minutes and 10seconds ,0.44 g of “P” was deposited at the cathode. Determine the charge on an ion of “P”(Faraday constant = 96500C)
Working:
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 0.5 x ((32 x 60) + 10)
= 965C
0.44 g of metal “P” are deposited by 965C
88g of of metal “P” are deposited by: 88 x 965= 193000 C
0.44
96500 C = 1 mole of electrons = 1 Faradays = single charge
193000 C -> 193000 = 2 moles/Faradays/charges => symbol of ion = P2+
96500
8. During purification of copper by electrolysis 1.48 g of copper was deposited when a current was passed through aqueous copper (II)sulphate(VI) for 2 ½ hours. Calculate the amount of current that was passed. (Cu= 63.5 ,1F = 96500C)
Working:
Equation at the cathode: Cu2+ (aq) + 2e-> Cu(s)
2 mole of electrons = 2 Faradays = 2 x 96500 C produce a mass =molar mass of copper thus;
63.5 g -> 2 x 96500C
1.48 g -> 1.48 x 2 x 96500 = 4255.1181 C
63.5
Current(I) in amperes = Quantity of electricity(in Coulombs) Time(t) in seconds
Substituting/converting time to second= 4255.1181C
(( 2 x 60) + 30) x60
= 0.4728 Amperes
17. Practically Faraday 1st law of electrolysis can be verified as below.
Verifying Faraday 1st law of electrolysis
Procedure.
Weigh clean copper plates electrodes. Record the masses of the electrodes in table I below. Place the electrodes in 1Mcopper(II) sulphate(VI) solution in a beaker. Set up an electrolytic cell.
Close the switch and pass a steady current of 2 amperes by adjusting the rheostat for exactly 20 minutes.Remove the electrodes from the electrolyte. Wash with acetone/ propanone and allow them to dry. Reweigh each electrode.
Sample results
| Mass of cathode before electrolysis | 7.00 g | Mass of anode before electrolysis | 7.75 g |
| Mass of cathode after electrolysis | 8.25 g | Mass of anode after electrolysis | 6.50 g |
| Change in mass at cathode after electrolysis | 1.25 g | Change in mass at anode after electrolysis | 1.25 g |
Answer the following questions:
I. Write the equation for the decomposition of the electrolytes during the electrolytic process.
H2O(l) -> OH– (aq) + H+(aq)
CuSO4(aq) -> SO42-(aq) + Cu2+(aq)
II. Name the ions in 1M copper(II) sulphate(VI) solution that are attracted/move to:
Cathode- Cu2+ (aq) fromcopper(II) sulphate(VI) solution and H+(aq) fromwater(H2O)
Anode– SO42-(aq) fromcopper(II) sulphate(VI) solution and OH– (aq) fromwater(H2O)
III. Write the equation for the reaction during the electrolytic process at the:
Cathode Cu2+ (aq) + 2e -> Cu(s)
Cu2+ ions are lower than H+ ions in the electrochemical series therefore selectively discharged at the cathode.)
Anode Cu (s) -> Cu2+(aq) + 2e
(Both OH– ions and SO42- ions move to the anode but none is discharged. The copper anode itself ionizes/dissolves/dissociate as less energy is used to remove an electron/ionize /dissociate copper atoms than OH– ions.
IV. Name the products of electrolysis of 1M copper(II) sulphate(VI) solution using copper electrodes.
Cathode-1.25 g of copper metal asbrown solid coat/deposits
Anode-1.25 g of copper metal erodes/decrease in size
V. (i)How many moles of electrons are used to deposit/erode one mole of copper metal at the cathode/anode?
From the equation at anode/cathode= 2 moles
(ii)How many Faradays are used to deposit/erode one mole of copper metal at the cathode/anode?
From the equation at anode/cathode : 2 moles = 2 Faradays
(iii)Calculate the quantity of electric charge used
Working:
Quantity of electricity (in Coulombs) = Current(I) x time(t)
Substituting /converting time to second = 2 x 20 x 60
= 2400C
VI. (i) Calculate the quantity of electricity required to deposit/erode one mole of copper at the cathode/anode(Cu=63.5)
Since 1.25 g of copper -> 2400C
Then 63.5 g (1mole of copper) -> 63.5 x 2400 = 121920 C
1.25
(ii)Determine the Faraday constant from the results in V(i) above
From the equation at;
Cathode Cu2+ (aq) + 2e -> Cu(s)
Anode Cu (s) -> Cu2+(aq) + 2e
2 moles = 2 Faradays -> 121920 C
1 moles = 1 Faradays -> 121920 = 60960 C
2
(iii) The faraday constant obtained above is far lower than theoretical.Explain
-high resistance of the wires used.
-temperatures at 25oC were not kept constant
-plates/electrodes used were not made of pure copper
-plates/electrodes used were not thoroughly clean copper
Further practice
1.An element P has a relative atomic mass of 88. When a current of 0.5 amperes was passed through the fused chloride of P for 32 minutes and 10 seconds, 0.44g of P were deposited at the cathode. Determine the charge on an ion of P. (1 faraday = 96500 Coulombs).
2.During electrolysis of aqueous copper (II) sulphate, 144750 coulombs of electricity were used. Calculate the mass of copper metal that was obtained
(Cu = 64 ;1 Faraday = 96500 coulombs) ( 3 mks)
3.A nitrate of a metal M was electrolysed .1.18 g of metal was deposited when a current of 4 ampheres flow for 16 minutes.Determine the formula of the sulphate(VI)salt of the metal.
(Faraday constant = 96500 , RAM of X = 59.0)
Working
Q = It =>( 4 x 16 x 60) = 3840 C
1.18 g of X => 3840 C
59.0 g => 59.0 x 3840 = 192000 C
1.18
96500 C = 1Faraday
192000 C= 192000 C x1 = 2F thus charge of M = M2+
96500 C
Valency of M is 2 thus formula of sulphate(VI)salt MSO4
4. Below is the results obtained when a current of 2.0ampheres is passed through copper(II)sulphate(VI)solution for 15 minutes during electrolysis using copper electrode.
Initial mass of cathode = 1.0 g
Final mass of cathode = 1.6 g
Change in mass of cathode = 0.60 g
(i)Determine the change in mass at the anode. Explain your answer.
Mass decrease = 0.6g.
Electrode ionization take place where the cathode increase in mass form the erosion of the anode
(ii)Calculate the quantity of electricity required to deposit one mole of copper.(Cu =63.5)
Q =It => 2 x 15 x 60 = 1800 coulombs
Method 1
0.60 g of copper ->1800 coulombs
63.5 g -> 63.5 x 1800 = 190500 Coulombs
0.60
Method 2
Moles of Copper = Mass => 0.60 = 9.4488 x10 -3 moles
Molar mass 63.5
9.4488 x10 -3 moles -> 1800 coulombs
1 Mole -> 1 x 1800 coulombs = 190500.381 coulombs
9.4488 x10 -3 moles
(iii)Determine the oxidation number of copper produced at the cathode and hence the formula of its nitrate (V)salt (1 Faraday = 96500 Coulombs)
96500 Coulombs -> 1 Faraday
190500.381 coulombs -> 190500.381 coulombs x 1
96500 Coulombs
= 1.9741 Faradays => 2F(whole number)
Charge of copper = 2+ = Oxidation number
=> Valency of copper = 2 hence chemical formula ofnitrate (V)salt = Cu (NO3)2
