CHEMISTRY OF CHLORINE
A.CHLORINE
Chlorine is a non-metallic element in group VII (Group 17) of the periodic table. It has electronic configuration 2:8:7. It gains one valence election to form stable Cl–ion, it belongs to the chemical family of halogens.
Occurrence
-As Brine-concentration sodium chloride solution dissolved in salty seas water, oceans and lakes e.g. Lake Magadi in Kenya is very salty.
-As rock-salt solid sodium chloride crystals in the earths crust all over the world.
B) Preparation
Chlorine gas may be prepared in the school laboratory from the following:
a)Heating solid Manganese (iv) Oxide and Concentrated Hydrochloric acid.
b) Heating Lead (IV) Oxide and concentrated hydrochloric acid.
c)Reacting Potassium Manganate (VII) with concentrated Hydrochloric acid
d)Reacting Potassium /sodium Dichromate (VI) Acid with Concentrated Hydrochloric acid.
Set up of school laboratory preparation of chlorine.
c) Properties of chlorine. (Questions)
1. What is the colour of chlorine?
Pale green.
2. Describe the smell of chlorine.
Pungent irritating smell.
3. What method is used in collection of chlorine gas explain.
-Downward delivery.
-Chlorine is 11/2 denser than air.
4.(i) What is the purpose of concentrated sulphuric (VI) acid.
-To dry the gas.
(ii) Name two other substances that can be used in place of concentrated sulphuric (VI) acid.
-Calcium chloride
-Silica gel
(iii) Name a substance that cannot be used in place of concentrated sulphuric (VI) acid explain.
-Calcium oxide reacts with chlorine.
5.(a)Write three possible reactions between concentrated hydrochloric acid and the oxidizing agents.
- 2KMnO4(s) +16HCl(aq) → 2KCl(aq)+2MnCl2(aq) + 8H2O(l) + 5Cl2(g)
2.K2Cr2O7(s) +14HCl(aq) → 2KCl(aq) + 2CrCl3(aq) + 7H2O(l) + 3Cl2(g)
3.Na2Cr2O7(s)+ 14HCl(aq) → 2NaCl(aq) + CrCl3(aq)+ 7H2O(l) + 3Cl2(g)
4.PbO2(s) + 4HCl(aq) → PbCl2(aq) + Cl2(g) + 2H2O(l)
5.MnO2(s)+ 4HCl(aq) → MnCl2(aq) + Cl2(g) + 2H2O(l)
(b) Why is Hydrochloric acid used in all the above cases?
Oxidizing agents KMnO4/PbO2/MnO2/K2Cr2O/Na2Cr2O7 readily oxidize hydrochloric acid to chlorine themselves reduced to their chlorides.
Generally:
2HCl (aq) + [O] → Cl2 (g) + H2O (l)
(From oxidizing agent)
6. State and explain the observation made when chlorine is bubbled in water.
Observation
-Pale yellow colour of chlorine fades.
-yellow solution formed.
Explanation
Chlorine dissolves then reacts with water to form yellow chlorine water. Chlorine water is chemically a mixture of hydrochloric acid and chloric(I)acid (hypochlorous acid).
A mixture of hydrochloric acid and chloric(I)acid (hypochlorous acid) is commonly called Chlorine water
Chemical equation:
Cl2(g) + H2O(l) → HCl(aq) + HClO(aq)
7. Chlorine water in a boiling tube inverted into a trough was exposed to sunlight for two hours. Using a well labeled diagram show and explain the observations made.
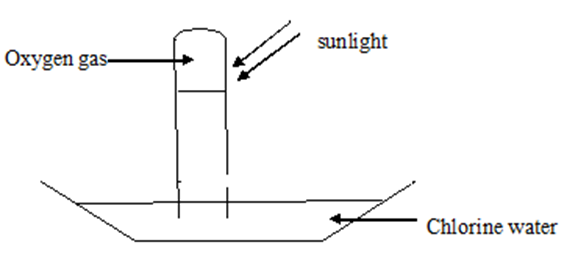
Chlorine (I) acid is an unstable compound.
After two hours the chloric (I) acid in chlorine water decomposes to hydrochloric acid and releases oxygen gas. This reaction takes place in sunlight.
Chemical equation
2HOCl(aq) → 2HCl(aq) + O2 (g)
8. State and explain the observation made when chlorine gas is bubbled in gas jar containing damp/wet/moist litmus papers.
Observation
The blue litmus turns red then both the red/blue litmus papers are bleached/decolourized.
Explanation
Chlorine reacts with water in the litmus papers to form acidic hydrochloric acid and chloric (l) acid that turns blue litmus papers red.
Chemical Equation
Cl2(g) + H2O(l) → HCl(aq) + HClO(aq)
Explanation
Unstable chloric (I) acid oxidizes the dye/colured litmus paper to colourless material
Chemical Equation
HClO(aq) + dye → HCl(aq) + (dye + O)
(coloured) (colourless)
Or:
HClO(aq) + dye-O → HCl(aq) + dye
(coloured) (colourless)
NB Chlorine does not therefore bleach/decolourize dry litmus paper/dye because chloric(I) acid cannot be formed in absence of water.
9. Blue litmus papers were put in a flask containing cold dilute sodium hydroxide. Chlorine gas was bubbled into the solution. State and explain the observations made.
Observation
blue litmus papers were bleached /decolorized.
Pale green colour of chlorine fades.
Explanation
-Sodium hydroxide reacts with chlorine to form sodium chloride and sodium hypochlorite. Sodium hypochlorite bleaches dyes by oxidation.
Chemical Equation
Cl2 + 2NaOH(aq) → NaCl(aq) + NaClO(aq) + H2O
NaClO(aq) + dye → NaCl(aq) + (dye + O)
(coloured) (Colourless)
NaClO(aq) + (dye-O) → NaCl(aq) + dye
(Coloured) (Colourless)
10.Blue litmus papers were put in flask containing hot concentrated sodium hydroxide. Chlorine gas was bubbled into the solution. State and explain the observations made.
Observation.
blue litmus papers were bleached.
Pale green colour of chlorine fades.
Explanation
Hot concentrated sodium hydroxide reacts with chlorine to form sodium chloride and sodium chloride (V).Sodium chlorate (V) bleaches by oxidation.
Chemical equation
2Cl2(g) + 4NaOH(aq) → 3NaCl(aq) + NaClO3(aq) + H2O(l)
NaClO3(aq) + 3(dyes) → NaCl(aq) + 3(dye + O)
NaClO3(aq) + 3(dyes-O) → NaCl(aq) + 3 dyes
NaClO3 is also a weed killer
11. State three main use of chlorine gas.
-Manufacture of polyvinyl chloride (P.V.C) // polychloroethene pipes.
-Manufacture of hydrochloric acid used in “Pickling” of metals.
-Manufacture of bleaching agents
-Chlorination of water to kill germs.
12. The diagram below shows the effect of chlorine on heated iron wool.
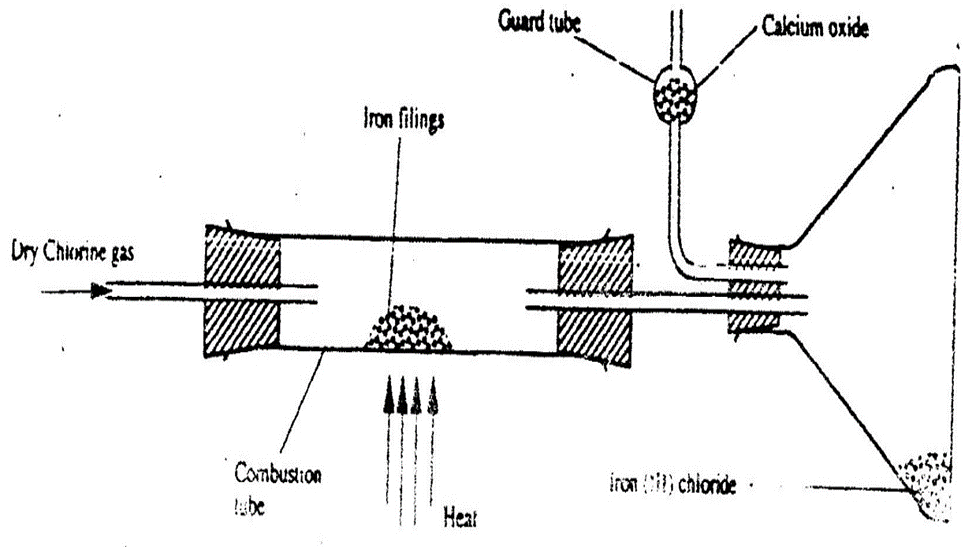
a) Identify a suitable drying agent to dry chlorine gas.
-Conc. H2SO4 / Concentrated sulphuric (VI) acid.
-Anhydrous Calcium (II) Chloride.
-Silica gel
b) State and explain the observations made in combustion tube in method I and II
Observation
Iron glows red hot
Brown crystals are formed
Explanation
Iron reacts with chlorine to form dark brown crystals of iron (III) Chloride.
This reaction is exothermic and requires no farther heating once started.
Iron (III) Chloride sublimes away ensuring the unreacted Iron completely reacts with chlorine gas.
Chemical equation
2Fe(s) + 3Cl2 (g) → 2FeCl3(g)
c) (i) Why is the brown solid collected at the point as shown in method I and II.
-Heated iron (III) Chloride crystals sublime to gas and solidify on the cooler parts.
(ii) Name another metal that can be used in place of iron to react with chlorine and collected at similar point on heating explain.
Metal Aluminum
Explanation
Aluminum reacts with chlorine to form a while sublimate of aluminum (III) chloride at the cooler parts
Chemical equation
2Al(s) + 3Cl2(g) → 2AlCl3(s/g)
d) What is the purpose of suction pump?
To pull the gaseous products into the set up.
e) What is the function of:
(i) Sodium hydroxide in method II. Explain.
To absorb poisonous/toxic excess unreacted chlorine gas.
Sodium hydroxide reacts with chlorine to form sodium chloride, Sodium hypochlorite and water.
Chemical equation:
2NaOH(aq) + Cl2(g) → NaCl(aq) + NaClO(aq) + H2O(l)
2KOH(aq) + Cl2(g) → KCl(aq) + KClO(aq) + H2O(l)
(ii) Anhydrous calcium chloride/calcium oxide in method I. Explain.
To absorb moisture/water in the set up to prevent it from hydrolyzing iron (III) chloride/aluminium oxide.
Explanation
Iron (III) chloride and Aluminium chloride fumes and reacts with small traces of water to form a solution of iron (III) hydroxide/aluminium hydroxide and hydrogen chloride gas.
Chemical equation
FeCl3(s) + 3HCl(aq) → Fe(OH)3(aq) + 3HCl(g)
AlCl3(s) + 3HCl(aq) → Al(OH)3(aq) + 3HCl(g)
f) Based on e (i) and (ii) above what precaution should be made in:
(i) method II to ensure correct results.
-Tube B should be completely dry to prevent hydrolysis of iron (III) Chloride to iron (III) hydroxide.
(ii) Carrying out method I
-Should be done in a fume chamber or in the open because chlorine gas is poisonous/toxic.
(g) Name another substance that can be used place of Sodium hydroxide in method I
Potassium hydroxide
(h) Calcium oxide cannot be used in place of calcium chloride during preparation of chlorine. Explain.
Calcium oxide is a base. It reacts /absorbs water to form calcium hydroxide solution.
Calcium hydroxide reacts with chlorine to form a mixture of calcium chloride and calcium hypochlorite.
Chemical equation
2Ca (OH)2(aq) + 2Cl2(g) → CaCl2(aq) + CaOCl2(aq) + H2O(l)
13. (a)State and explain the observation made when a piece of burning magnesium ribbon is lowered in a gas jar containing chlorine gas.
-Magnesium ribbon continues burning with a bright flame.
-White solid formed.
-Pale yellow colour of chlorine fades
Explanation:
Magnesium reacts with chlorine forming a white solid of magnesium chloride.
Chemical equation
Mg(s) + Cl2(g) → MgCl2(s)
(b) Write the equation for the reaction that takes place if zinc is used.
Zn(s) + Cl2(g) → ZnCl2(s)
14. Burning phosphorus was lowered in a gas jar containing chlorine gas.
a) State the observations made.
-Phosphorus continues to burn.
-Dense white fumes formed.
-Pale green colour of chlorine fades.
b) Write two possible equations that take place.
P4(s) + 6Cl2(g) → 4 PCl3(s)
P4(s) + 10Cl2(g) → 4 PCl3(s)
(c) State two reasons why the deflagrating spoon with rid/cover should be used.
-Chlorine in the gas jar is poisonous/toxic.
-Burning phosphorus produces poisonous/toxic phosphorus (III) chloride // phosphorus (V) chloride.
-Ensure the reaction is not affected by air/oxygen from the atmosphere.
(d) After the reaction is complete, 2cm3 of distilled water were added. The solution formed was tested with both blue and red litmus papers.
(i) State the observations made.
-Blue litmus paper turns red
-Red litmus paper remain red
(ii) Explain the observation made in d(i) above
-Phosphoric (V) Chloride hydrolyze in water to phosphoric (V) acid and produce hydrogen chloride gas. Both hydrogen chloride and phosphoric (V) acid are acidic.
Chemical equation
PCl5 (l) + 4H2O(l) → H3PO4 (aq) + 5HCl(g)
15. State and explain the observations made when gas jar containing chlorine is inverted over another containing hydrogen sulphide gas.
Observation
Yellow solid formed.
Pale colour of chlorine fades
Explanation
Chlorine oxidizes hydrogen sulphide to sulphur itself reduced to hydrogen chloride gas. A little water catalyzes the reaction.
Chemical equation
H2S(g) + Cl2(g) → S(s) + HCl(g)
(yellow solid) (White Fume)
16. Chlorine was bubbled in aqueous ammonia solution in a beaker state and explain the observation made.
Observation:
White fumes evolved.
Pale green colour of chlorine fades.
Explanation
Chlorine reacts with ammonia gas to form a dense white fume of ammonia chloride and Nitrogen gas is produced.
Chemical equation
8NH3(g) + 3Cl2(g) → 6NH4Cl(s) + N2(g)
17. (a) Dry gas was bubbled in cold dilute sodium hydroxide solution. Explain the observations made:
Observation
Pale green colour of chlorine fades.
Pale yellow solution is formed.
Explanation
Chlorine reacts withhot concentrated sodiumsodium hydroxide / Potassium hydroxide solution to form pale yellow solution of metal chlorate (V) and chlorides of the metal
Chemical equation
Cl2(g) + 2NaOH → NaClO(aq) + NaCl(aq) + H2O(l)
(sodium hydroxide) (Sodium Chlorate (I))
Cl2(g) + 2KOH → KClO(aq) + NaCl(aq) + H2O(l)
(Potassium hydroxide) (Potassium Chlorate (I))
(b)The experiment in 17(a) was repeated with hot concentrated sodium hydroxide solution. Explain the observation made.
Observation
Pale green colour of chlorine fades.
Pale yellow solution is formed.
Explanation
Chlorine reacts with hot concentrated Sodium hydroxide/Potassium hydroxide solution to form pale yellow solution of metal chlorate (v) and chlorides of metals.
Chemical equation
3Cl2(g) + 6NaOH(aq) → NaClO3 (aq) + 5NaCl(aq) + 3H2O(l)
(Sodium hydroxide) (Sodium Chlorate (V))
3Cl2(g) + 6KOH(aq) → KClO3 (aq) + 5KCl(aq) + 3H2O(l)
(Potassium hydroxide) (Potassium Chlorate (V))
The products formed when chlorine reacts with alkalis depend thus on temperature and the concentration of alkalis.
(c) (i) Write the equation for the formation of calcium chlorite (I) and calcium chlorate (V).
2Ca (OH)2(aq) + 2Cl2(g) → CaCl2(aq) + CaOCl2(aq) + H2O(l)
(Calcium hydroxide) (Calcium Chlorate(I))
(Cold/dilute)
Ca (OH)2(aq) + Cl2(g) → CaCl2(aq) + Ca(ClO3)2(aq) + H2O(l)
(Calcium Chlorate(V))
B: THE HALOGENS
a) What are halogens?
These are elements in group VII of the periodic table. They include:
| Element | Symbol | Atomic number | Electric configuration | Charge of ion | Valency | State at Room Temperature |
| Fluorine Chlorine Bromine Iodine Astatine | F Cl Br I At | 9 17 35 53 85 | 2:7 2:8:7 2:8:18:7 2:8:18:18:7 2:8:18:32:18:7 | F– Cl– Br– I– At– | 1 1 1 1 1 | Pale yellow gas Pale green gas Red liquid Grey Solid Radioactive |
b) Compare the atomic radius and ionic radius of chloride ion and chlorine. Explain.
The radius of chlorine is smaller than the ionic radius o the chloride ion.
Effective nucleus attraction on outer energy level in chloride ion is less than chlorine atom because of extra gained electron gained electron that repelled thus causes the outer energy level to expand/increase.
c) Compare the atomic radius of chlorine and fluorine Explain.
Atomic radius of Fluorine is smaller than that of chlorine.
Chlorine has more energy levels than fluorine occupied by more electrons.
d) Chlorine is a gas, Bromine is a liquid, Iodine is a solid. Explain the above observations.
–Bromine, Chlorine and iodine exists as diatomic molecules bonded by strong covalent bond. Each molecule is joined to the other by weak intermolecular forces/ Van-der-waals forces.
-The strength of intermolecular/Van-der-waals forces of attraction increase with increase in molecular size/atomic radius.Iodine has therefore the largest atomic radius and thus strongest intermolecular forces to make it a solid.
e) (i) What is electronegativity?
Electronegativity is the tendency/ease of acquiring /gaining electrons by an element during chemical reaction.
It is measured using Pauling’s scale.
Fluorine with Pauling scale 4.0 is the most electronegative element in the periodic table and thus the highest tendency to acquire/gain extra electron.
(ii) The table below shows the electronegativity of the halogens.
| Halogen | F | Cl | Br | I | At |
| Electronegativity (Pauling’s scale) | 4.0 | 3.0 | 2.8 | 2.5 | 2.2 |
Explain the trend in electronegativity of the halogens.
Decrease down the group from fluorine to Astatine
Atomic radius increase down the group decreasing electron – attracting power down the group from fluorine to astatine.
(f) (i)What is electron affinity
Electron affinity is the energy required to gain an electron in an atom of an element in its gaseous state.
(ii) Study the table below showing the election affinity of halogens for the process x + e → x–
| Halogen | F | Cl | Br | I |
| Electron affinity kJmole-1 | -333 | -364 | -342 | -295 |
(iii) Explain the trend in electron affinity of the halogens.
-Decrease down the group
-Atomic radius of halogens increase down the group thus incoming/gained electron is attracted less strongly by the progressively larger atoms with a decreasing effective nuclear charge on outer energy level
(iv) Which is a move stable ion Cl– or Br – explain?
-Cl– ion.
-Has a more negative/exothermic electron affinity than Br–
(v) Differentiate between electron affinity and:
I. Ionization energy.
Ionization energy is the energy required to lose /donate an electron in an atom of an element in its gaseous state while electron affinity is the energy required to gain/acquire extra electron by an atom of an element in its gaseous state.
Both are measured in kilojoules per mole.
II. Electronegativity.
-Electron affinity is the energy required to gain an electron in an atom of an element in gaseous state. It involves the process:
X(g) + e → X–(g)
Electronegativity is the ease/tendency of gaining/ acquiring electrons by an element during chemical reactions.
It does not involve use of energy but theoretical arbitrary Pauling’s scale of measurements.
(g) (i) 5cm3 of sodium chloride, Sodium bromide and Sodium iodide solutions were put separately in test tubes. 5 drops of chlorine water was added to each test tube: state and explain the observation made.
Observation
Yellow colour of chlorine water fades in all test tubes expect with sodium chloride.
-Coloured Solution formed.
Explanation
Chlorine is more electronegative than bromine and iodine. On adding chlorine water, bromine and Iodine are displaced from their solutions by chlorine.
(ii) The experiment in g (i) was repeated with 5 drops of bromine water instead of chlorine water .explain the observation made.
Observation
Yellow colour of bromine water fades in test tube containing sodium iodide.
Brown solution formed in test tube containing sodium iodide
Explanation
Bromine is more electronegative than iodide but less 6than chlorine.
On adding Bromine water, iodide displaced from its solution but not chlorine.
(iii) Using the knowledge in g(i) and (ii) above,
- Complete the table below using (X) to show no reaction and (√) to show a reaction.
| Halide ion Halogen ion in solution Halogen | F– | Cl– | Br– | I– |
| F2 | X | √ | √ | √ |
| Cl2 | X | X | √ | √ |
| Br2 | X | X | X | √ |
| I2 | X | X | X | √ |
Write an ionic equation for the reaction where there is (V)
F2 (g)+ 2Cl– (aq) -> 2F–(aq) + Cl2(g)
F2 (g)+ 2Br– (aq) -> 2F–(aq) + Br2(aq)
F2 (g)+ 2I– (aq) -> 2F–(aq) + I2(aq)
Cl2 (g) + 2Br– (aq) -> 2Cl–(aq) + Br2(aq)
Cl2 (g)+ 2I– (aq) -> 2Cl–(aq) + I2(aq)
Br2 (aq)+ 2I– (aq)- -> 2Br–(aq) + I2(aq)
(h) State one uses of:
- Fluorine
Manufacture of P.T.F.E (Poly tetra fluoroethene) synthetic fiber.
Reduce tooth decay when added in small amounts/equations in tooth paste.
Note: large small quantities of fluorine /fluoride ions in water cause browning of teeth/flourosis.
Hydrogen fluoride is used to engrave word pictures in glass.
- Bromine
Silver bromide is used to make light sensitive photographic paper/films.
- Iodide
Iodine dissolved in alcohol is used as medicine to kill bacteria in skin cuts. It is called tincture of iodine.
- The table below to show some compounds of halogens.
| Element Halogen | H | Na | Mg | Al | Si | C | P |
| F | HF | NaF | MgH2 | AlF3 | SiF4 | CF4 | PF3 |
| Cl | HCl | NaCl | MgCl | AlCl3 | SiCl3 | CCl4 | PCl3 |
| Br | HBr | NaBr | MgBr2 | AlBo3 | SiBr4 | CBr4 | PBr3 |
| I | Hl | Nal | Mgl2 | All3 | SiL4 | Cl2 | Pb3 |
(j) (i) Using dot (.) and Cross (x) to represent electrons, show the bonding in chlorine molecule.
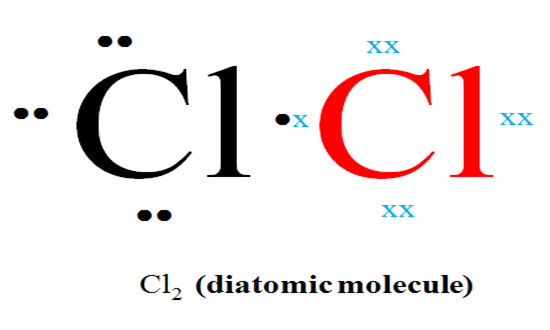
- Name the type of bond formed.
Covalent.
- Below is the table showing the bond energy of four halogens.
Bond Bond energy k J mole-1
Cl-Cl 242
Br-Br 193
I-I 151
- What do you understand by the term “bond energy”
Bond energy is the energy required to break/ form one mole of chemical bond
- Explain the trend in bond Energy of the halogens above:
–Decrease down the group from chlorine to Iodine
-Atomic radius increase down the group decreasing the energy required to break the covalent bonds between the larger atom with reduced effective nuclear charge an outer energy level that take part in bonding.
(k) Some compounds of chlorine are in the table below the oxidation state of chlorine in each compound.
Compound Oxidation state Name of compound
NaClO3 +5 Sodium chlorate (V)
ClO2 +4 Chloric (IV) oxide
KClO2 +3 Potassium chlorate (III)
NaClO +1 Sodium Chlorite (I)
Cl2 0 Chlorine Molecule
NaCl -1 Sodium Chloride (I)
MgCl2 -1 Magnesium Chloride (I)
C. HYDROGEN CHLORIDE
- Occurrence
Hydrogen Chloride does not occur free in the atmosphere or in nature
- Preparation
Hydrogen chloride may be prepared in the school laboratory by reacting solid sodium/potassium chloride crystals with concentrated sulphuric (Vi) acid as in the set up below.
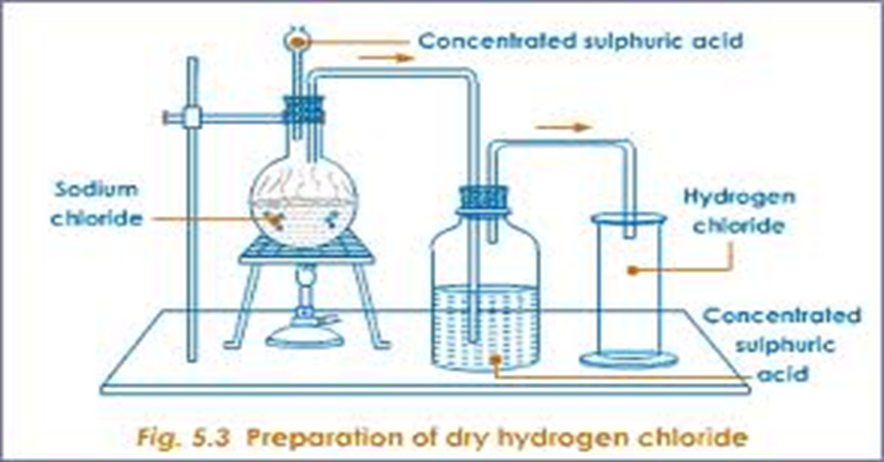
- Properties of hydrogen chloride gas(questions)
- What precautions should be taken when handling concentrated sulphuric acid? Explain.
-Wear protective clothing/gloves to avoid accidental contact with skin.
-Concentrated sulphuric (VI) acid is highly corrosive-it causes painful wounds when in contact with skin.
- What method of gas collection is used? Explain.
-Downward delivery// upward displacement of water
-Hydrogen chloride is denser than air.
- a) Write the equation for the reaction that takes place.
NaCl(s) + H2SO4(l) -> NaHSO4(aq) + HCl(g)
KCl(s) + H2SO4(l) -> KHSO4(aq) + HCl(g)
NaCl is commonly used because it is cheaper than KCl
b) What property of concentrated sulphuric (VI) acid is used during the above reaction
-is the least volatile mineral acid, thus displace the more volatile hydrogen chloride from its salt (KCl/NaCl)
- i)What is the purpose of concentrated sulphuric (VI) acid.
-Drying agent / to dry the gas.
ii) What property of concentrated sulphuric (VI) acid is used during the above use.
-Is hygroscopic – absorbs water but do not form solution.
iii) Name another substance which can be used to dry chlorine gas.
-anhydrous Calcium chloride
– silica gel
iv)Using a chemical equation, explain why anhydrous calcium oxide cannot be used in flask B
-Calcium oxide reacts with water /moisture to form calcium hydroxide. The calcium hydroxide formed reacts with chlorine to form calcium hypochlorite.
Chemical equations:
CaO(s) + 2H2O(l) -> Ca(OH)2(aq) + H2O(l)
Ca(OH)2(aq) + Cl2 (g) -> CaOCl2(aq) + H2O(l)
This reduces the amount of Chlorine produced.
d)Blue and red litmus papers were dipped in the hydrogen chloride prepared above. The Procedure was repeated with damp/wet/moist litmus papers. Explain the differences in observations made.
–Dry blue litmus papers remain blue
-Dry red litmus papers remain red
-Damp/moist/wet blue litmus papers turn red
-Damp/moist/wet red litmus paper turns red.
-Dry hydrogen chloride is a molecular compound that is joined by covalent bonds between the atoms. The gas is polar thus dissolves in water and ionize completely to free H+ that are responsible to turning blue litmus paper red.
- Dry hydrogen chloride gas was bubbled in two separately beakers containing water and in methylbenzene.
- Classify the two solvents as either “polar” or “non-polar”
Water – polar
Methylbenzene – non-polar
(ii) State and explain the observations made in the beaker containing:
(i)Methylbenzene
Colour of litmus solution remain.
Hydrogen chloride is a molecular substance. When dissolved in non-polar solvent, it does not dissociate / ionize to release H+ ions that changes the colour of litmus solution.
(ii)Water
Colour of litmus solution change to red.
Hydrogen chloride is a molecular substance. When dissolved in polar solvent like water, it dissociate/ionize to release H+ ions that changes litmus solution to red.
(iii)Why should an inverted filter funnel be used to dissolve hydrogen chloride.
– The filter funnel is dipped just below the water surface to increase the surface area of dissolving the gas and prevent suck back.
(iv)Name the solution formed when hydrogen chloride dissolves in water.
Hydrochloric acid
(f) Describe the test for presence of hydrogen chloride gas.
-Dip a glass rod in ammonia. Bring it to the mouth of a gas jar containing a gas suspected to be hydrogen chloride
-White fumes of ammonia chloride are formed.
(g) Place 5cm3 of dilute hydrochloric acid into a four separate test tubes. To separate test tube add zinc, magnesium iron and copper metals. State and explain the observations made.
Observation
– Effervescence/bubbles/fizzing in all cases except copper
- Colourless solution formed with zinc and magnesium.
- Green solution formed with ion.
- Gas produced that extinguishes splint with explosion.
Explanation
Metals above hydrogen in reactivity series react with hydrochloric and liberating hydrogen gas.
Chemical Equation:
Concentrated hydrochloric acid is a weak oxidizing agent than other concentrated acids i.eSulphuric (VI) acid and nitric (V) acid that react with all metals even those lower in the reactivity series.
(h) Place 5cm3 of dilute hydrochloric acid into five separate test tubes. To separate test tubes, add calcium carbonate, silver carbonate, copper carbonate, iron (II) carbonate and Sodium hydrogen carbonate. Explain the observations made.
Observation
Effervescence/bubbles/fizzing vigorously except in silver carbonate and lead (II) carbonate that stop later.
- Colourless solution formed except with iron (II) carbonate and copper (II) carbonate
- Green solution formed with iron (II) carbonate
- Blue solution formed with copper (II) carbonate
Explanation.
Carbonates and hydrogen carbonate react with dilute hydrochloric acid to produce carbon (IV) oxide, water and form chlorides.
All chlorides formed are soluble Except Lead (II) Chloride (soluble on heating/warming) and silver chloride.
Chemical equation:
CaCO3 (s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
(Colourless solution)
Chemical equation:
Ag2CO3 (s) + 2HCl(aq) → AgCl(s) + H2O(l) + CO2(g)
(Coats/Cover Ag2CO3)
Chemical equation:
CuCO3 (s) + 2HCl(aq) → CuCl2(aq) + H2O(l) + CO2(g)
(Blue Solution)
Chemical equation:
FeCO3 (s) + 2HCl(aq) → FeCl2(aq) + H2O(l) + CO2(g)
Chemical equation:
NaHCO3 (s) + HCl(aq) → NaCl(aq) + H2O(l) + CO2(g)
Place 5cm3 of dilute sodium hydroxide, Potassium hydroxide and aqueous ammonia solution into three separate test tubes. Add one drop of phenolphthalein indicator drop wise, add dilute hydrochloric acid. Explain the observations made.
Observation
Colour of Phenophthalein indicator change from pink to colourless.
Explanation
Hydrochloric acid neutralizes alkalis to salt and water
When all the alkali has reacted with the acid, An extra slight excess acid turns the indicator used to colourless.
Chemical equation:
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Chemical equation:
KOH(aq) + HCl(aq) → KCl(aq) + H2O(l)
Chemical equation:
NH4OH(aq) + HCl(aq) → NHaCl(aq) + H2O(l)
(j) Place 5cm3 of hydrochloric acid into four separate test tube tubes Separately add about 1g of each of copper (II) Oxide, Zinc (II) Oxide, Lead (II) Oxide< Calcium (II) Oxide. What happens to each test tube? Explain.
Observation:
All Solid dissolves except Lead (II) Oxide
Colourless solution formed with zinc Oxide and calcium (II) Oxide blue solution formed with copper (II) Oxide.
Explanation:
Metal oxides dissolves in dilute hydrochloric acid to form water and chloride salt Insoluble Lead (II) chloride and silver chloride once formed cover/coat unreacted oxides stopping further reaction.
Chemical equation: CuO(s) + HCl (aq) → CaCl2(aq) + H2O(l)
Chemical equation: CaO(s) + HCl (aq) → CaCl2 (aq) + H2O (l)
Chemical equation: PbO(s) + 2HCl (aq) → PbCl2 (aq) + H2O (l)
Chemical equation: ZnO(s) + HCl (aq) → ZCl2 (aq) + H2O (l)
(k) Manufacture of Hydrochloric acid.
(i) Raw Materials
1. Hydrogen
(i) During electrolysis of Brine from the flowing mercury-cathode cell during the manufacture of sodium hydroxide solution.
(ii)From water gas by passing steam in heated charcoal.
C(s) + H2O → CO(g) + H2(g)
(iii)From partial oxidation of natural gas/methane
CH4(g) + O2(g) → CO(g) + 3H2(g)
2.Chlorine
(i)From electrolysis of fused/solid sodium chloride in the downs process during extraction of sodium
(ii)From electrolysis of brine/concentrated sodium chloride solution in the flowing mercury-cathode during the manufacture of sodium hydroxide solution.
(ii)Chemical processes.
Hydrogen and chlorine gases are separately passed through concentrated sulphuric(VI) acid to act as a drying agent.
Small amount of pure hydrogen is continuously ignited in a chamber with continous supply of pure dry chlorine.
Large amount of hydrogen explodes.
Hydrogen burns in chlorine to form hydrogen chloride gas.
Chemical Equation
H2(g) + Cl(g) → 2HCl(g)
The hydrogen chloride produced is then passed up to meet a downward flow of water in the absorbtion chambers. Hydrogen chloride is very soluble in water and dissolves to form 35% concentrated hydrochloric acid.
Chemical Equation
HCl(g) + (aq) → HCl(aq)
The absorption chamber is shelved and packed with broken glass beads to
(i)Slow down the downward flow of water.
(ii)Increase surface area over which the water dissolves
The hydrochloric acid is then transported in steel tanks lined with rubber for market
(iii)Uses of Hydrochloric Acid
- To standardize the pH of (alcohol and wines)
- Regenerating ion-exchange resin during removal of hardness of water.
- Pickling of metals to remove oside layers on their surfaces.
- In the manufacture of dyes and drugs.
- Making zinc chloride for making dry cells.
(ii)Environmental effects of manufacturing HCl
- Hydrochloric acid is acidic. Any leakage from a manufacturing plant to nearby rivers/lake causes exess acidity that lowers pH of water killing marine life.
- Hydrogen chloride leakage into atmosphere dissolves to form “acidic rain” that accelerate corrosion in buildings, Breathing problems to human beings and kill fauna and flora around the paint.
- Chlorine leakage causes breathing and sight problems to human being. It accelerates bleaching of dyed metals.
- Hydrogen leakage can cause an explosion because impure hydrogen explodes on ignition.
- Factors considered in setting hydrochloric acid manufacturing plant.
- Nearness to the manufacturing of sodium hydroxide because the byproducts of electrolysis of brine are the raw materials for hydrochloric acid plant.
- Availability of natural gas for extraction of hydrogen.
- Nearness/Availability of water to dissolve the hydrogen chloride gas.
- Availability of labour, market, capital and good means of transport.
D: CHLORIDE (Cl–) SALTS
- Occurrence.
- Chlorides are salts derived from hydrochloric acid. Hydrochloric acid is a monobasic (HX) salt with only one ionazable/replaceable “H” in its molecule. All chlorides are therefore normal salts.
- All metals exist as chloride salt except platinum and gold as below
| Metal | K | Na | Li | Mg | Ca | Al | Zn | Fe | Pb | H. | Cu | Ag | Hg |
| Formula of chloride | KCl | NaCl | LiCl | MgCl2 | CaCl2 | AlCl3 | ZnCl2 | FeCl2 FeCl3 | PbCl PbCl4 | HCl | CuCl CuCl2 | AgCl | Hg2Cl2 HgCl2 |
(i)Both FeCl2 and FeCl3 exists but FeCl2 is readily oxidized to FeCl3 because it is more stable.
(ii)PbCl2 and PbCl4 exist but PbCl4 is only oxidized to form PbCl2 by using excess chlorine. It is less stable.
(iii)CuCl and CuCl2 exists but CuCl2 is (thermodynamically) more stable than CuCl. CuCl disproportionate to Cu and CuCl2..
(iv)HgCl and HgCl2exists as molecular compounds.
- All chlorides are soluble/dissolves in water except silver chloride(AgCl), Copper (I) chloride CuCl, Mercury (I) Chloride Hg2Cl2 and Lead (II) Chloride PbCl2 that dissolves in warm water.
- Most chlorides are very stable compounds. They do not decompose on gentle or strong bunsen burner heating in a school laboratory except Ammonium Chloride.
- Heating ammonium chloride
Place about 2g of solid ammonium chloride crystals in a clean dry boiling tube.Heat gently then strongly.
Observation
–red litmus paper turn blue
-blue litmus paper remains blue
Then later:
-both blue litmus papers turn red
Explanation:
Ammonium chloride on heating decomposes through chemical sublimation to ammonia and hydrogen chloride gas. Ammonia gas is less dense than hydrogen chloride. It is a basic gas and diffuses out faster to turn red litmus paper to blue. Hydrogen chloride is an acidic gas .It is denser than ammonia gas and thus diffuses slower than ammonia gas to turn the already both blue litmus paper to red.
Chemical equation
NH4Cl(s) -> HCl(g) + NH3 (g)
(acidic gas) (basic/alkaline gas)
- Test for Cl– ions
- The following experiment shows the test for the presence of Cl– ions in solids chloride salts.
- Procedure:
Place about 1g of sodium chloride, Zinc chloride and copper (II) chloride in separate boiling tubes. Place moist blue and red litmus papers on the mouth of the test tube. Carefully, add three drops of concentrated sulphuric (VI) acid.
Dip a glass rod in aqueous ammonia solution then bring it to the mouth of the boiling tube.
| observation | inference |
| -red litmus paper remain red -blue litmus paper turn red -vigorous effervescence/fizzing /bubbling -white fumes produced on | H+ ions Cl– ions HCl gas suspected |
(b)Explanation:
Concentrated sulphuric (VI) acid is the less volatile mineral acid.
It vigorously displaces chlorine in metal chlorides to evolve acidic hydrogen chloride gas fumes.
Chemical equation
NaCl(s) + H2SO4(l) -> NaHSO4(aq) + HCl(g)
KCl(s) + H2SO4(l) -> KHSO4(aq) + HCl(g)
CuCl2(s) + H2SO4(l) -> CuSO4(aq) + 2HCl(g)
ZnCl2(s) + H2SO4(l) -> ZnSO4(aq) + 2HCl(g)
Hydrogen chloride and ammonia gases react and form white fumes of ammonium chloride that confirms presence of Cl– ions in the solid substance.
Chemical equation
NH3(g) + HCl(g) -> NH4Cl(s)
- The following experiment shows the test for the presence of Cl– ions in solution /aqueous chloride salts.
(i)Using aqueous Lead (II) nitrate(V)
(a)Procedure:
I.Place about 5cm3 of sodium chloride, Iron (III) chloride and copper (II) chloride in separate boiling tubes. Add four drops of Lead (II) nitrate(V) solution to each. Preserve.
| Observation | Inference |
| White precipitate/ppt | SO42-, SO32-, Cl–,CO32- |
II.To the preserved sample, add six drops of nitric (V) acid. Preserve.
| Observation | Inference |
| White precipitate/ppt persist | SO42-, Cl– |
III. To the preserved sample, heat the mixture to boil
| Observation | Inference |
| White precipitate/ppt dissolves on boiling/warming | Cl– |
Explanation:
I.When Lead(II) nitrate(V) solution is added to an unknown salt , a white precipitate/ppt of Lead(II) sulphate(VI) Lead(II) carbonate(IV) Lead(II) sulphate(IV) Lead(II) chloride(I) are formed.
Ionic equation:
Pb2+ (aq) + SO42-(aq) -> PbSO4(s)
Pb2+ (aq) + SO32-(aq) -> PbSO3(s)
Pb2+ (aq) + CO32-(aq) -> PbCO3(s)
Pb2+ (aq) + Cl–(aq) -> PbCl2(s)
II.When the white precipitate/ppt formed is acidified with dilute nitric(V) acid, the white precipitate of Lead(II) sulphate(VI) and Lead(II) chloride(I) persist/remain while that of Lead(II) carbonate(IV) and Lead(II) sulphate(IV) dissolves.
III.On heating /warming Lead (II) chloride (I) dissolves but on cooling it recrystallizes.This shows the presence of Cl– ions in aqueous solutions
. (ii)Using aqueous silver (I) nitrate(V)
Procedure
I. Place about 5cm3 of sodium chloride, Iron (III) chloride and copper (II) chloride in separate boiling tubes. Add four drops of silver(I) nitrate(V) solution to each. Preserve.
| Observation | Inference |
| White precipitate/ppt | Cl–, CO32- |
II. To the preserved sample, add six drops of nitric (V) acid. Preserve.
| Observation | Inference |
| White precipitate/ppt persist | Cl– |
Explanation:
I.When silver(I) nitrate(V) solution is added to an unknown salt , a white precipitate /ppt of silver(I) carbonate(IV) and silver(I) chloride(I) are formed.
Ionic equation:
2Ag+ (aq) + CO32-(aq) -> Ag2CO3(s)
Ag+ (aq) + Cl–(aq) -> AgCl(s)
II. When the white precipitate/ppt formed is acidified with dilute nitric (V) acid, the white precipitate of silver (I) chloride (I) persist/remain. This shows the presence of Cl–ions in aqueous solutions.
Silver (I) carbonate (IV) dissolves when reacted with nitric (V) acid.
