COMPREHENSIVE REVISION QUESTIONS
1. In an experiment, dry hydrogen chloride gas was passed through heated zinc turnings as in the set up below. The gas produced was the passed through copper(II) oxide
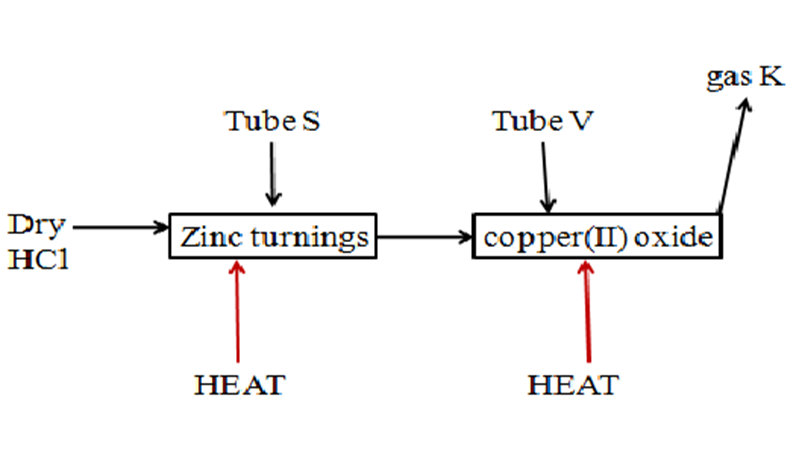
- Write the equation for the reaction :
(i)For the school laboratory preparation of hydrogen chloride gas.
NaCl(s) + H2SO4(l) -> NaHSO4(aq) + HCl(g)
(ii)in tube S
Zn(s) + 2HCl(aq) -> ZnCl2(aq) + H2(g)
b)State and explain the observation made in tube V.
Observations-colour of solid changes from black to brown
-colourless liquid forms on the cooler parts of tube V
Explanation-Hydrogen produced in tube S reduces black copper(II) oxide to brown copper metal and the gas oxidized to water vapour that condense on cooler parts..
Chemical equation.
CuO(s) +H2(g) ->Cu(s) + H2O(l)
(c)How would the total mass of tube S and tube V and their contents compare before and after the experiment.
Tube S- Mass increase/rise because Zinc combine with chlorine to form heavier Zinc Chloride.
Tube V- Mass decrease/falls/lowers because copper (II) oxide is reduced to lighter copper and oxygen combine with hydrogen to form water vapour that escape.
2. Chlorine is prepared by using solid sodium chloride, concentrated sulphuric(VI) acid and potassium manganate(VII)
a)What is the role of the following in the reaction;
(i) concentrated sulphuric(VI)
To produce hydrogen chloride gas by reacting with the solid sodium chloride.
(ii) Potassium manganate(VII)
To oxidize hydrogen chloride gas to chlorine
3.Use the flow chart below to answer the questions that follow.
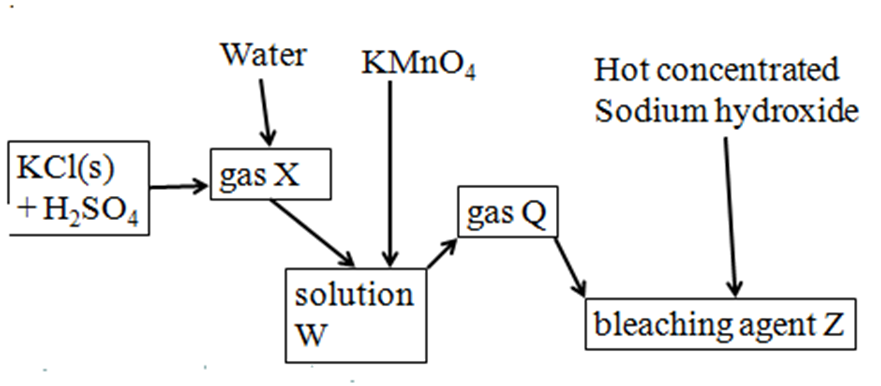
a)(i) Name:
gas X Hydrogen chloride
solution W hydrochloric acid
gas Q chlorine
bleaching agent Z sodium chlorate(V)
b)Write the chemical equation for the formation of :
(i) gas X
NaCl(s) + H2SO4(l) -> NaHSO4(aq) + HCl(g)
(ii)solution W
HCl(g) + (aq) -> HCl(aq)
(iii)gas Q
2KMnO4 + 16HCl(aq) -> 2KCl(aq) + 2MnCl2(aq) + 8H2O(l) + 5Cl2(g)
(iv)bleaching agent Z
6NaOH(aq) + 3Cl2(g) ->NaCl(aq) + NaClO3(aq) + 3H2O(l)
c)State and explain the following observations;
(i) a glass rod dipped in aqueous ammonia is brought near gas X
Observation: Dense white fumes
Explanation:Ammonia gas reacts with hydrogen chloride gas to form dense white fumes of ammonium chloride.
Chemical equation: NH3(g) +HCl(g) -> NH4Cl(s)
(ii)Wet blue and red litmus papers were dipped into gas Q
Observations: Blue litmus paper turned red the both are bleached
/decolorized.
Explanations: chlorine reacts with water to form both acidic hydrochloric and chloric (I) acids that turn blue litmus paper red. Unstable chloric (I) acid oxidizes the dye in the papers to colourless.
Chemical equations
Cl2(g) + HCl(aq) ->HCl(aq) + HClO(aq)
Coloured dye +HClO(aq) ->HCl(aq) + (Colourless dye +O)//
(Coloured dye-O) + HClO(aq) ->HCl(aq) + Colourless dye
4.Use the flow chart below to answer the questions that follow
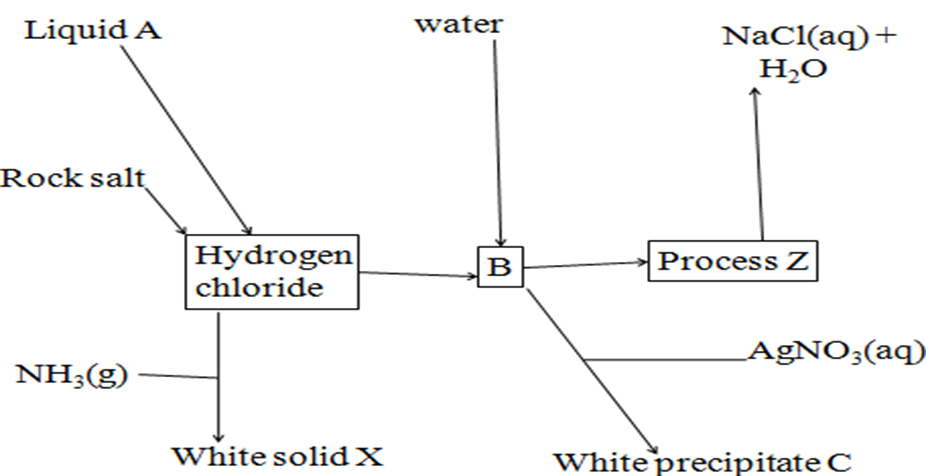
- Name
Liquid A Concentrated sulphuric(VI) acid
Process Z Neutralization
White solid X Ammonium chloride
b)Write the equation for the formation of:
(i) Hydrogen chloride
NaCl(s) + H2SO4(l) -> NaHSO4(aq) + HCl(g)
(ii) B
HCl(g) + (aq) -> HCl(aq)
(iii)process Z (using ionic equation)
H+ (aq) + OH–(aq) -> H2O(l)
(iv)C (using ionic equation)
Ag+ (aq) + Cl–(aq) -> AgCl(s)
c)Describe how solution B is obtained.
Bubbling hydrogen chloride gas through inverted funnel into distilled water until no more dissolve.
5 The results obtained when halogens are bubbled into test tubes containing solutions of halide A,B and C is as in the table below. Tick(v) means a reaction took place. Cross(x) means no reaction took place.
| Halogens | Halide ions in solution | ||
| A | B | C | |
| I2 | x | – | x |
| Br2 | x | v | – |
| Cl2 | – | v | v |
a)Identify the halide ions represented by letter
A Cl–
B I–
C Br–
b)Write the ionic equation for the reaction that take place with halide:
(i) C
Cl2(g) + 2Br–(aq) -> 2Cl–(aq) + Br2(aq)
(ii) B
Cl2(g) + 2Br–(aq) -> 2Cl–(aq) + Br2(aq)
Cl2(g) + 2I–(aq) -> 2Cl–(aq) + I2(aq)
6.The diagram below shows a set up of apparatus for the school laboratory collection of dry chlorine gas.
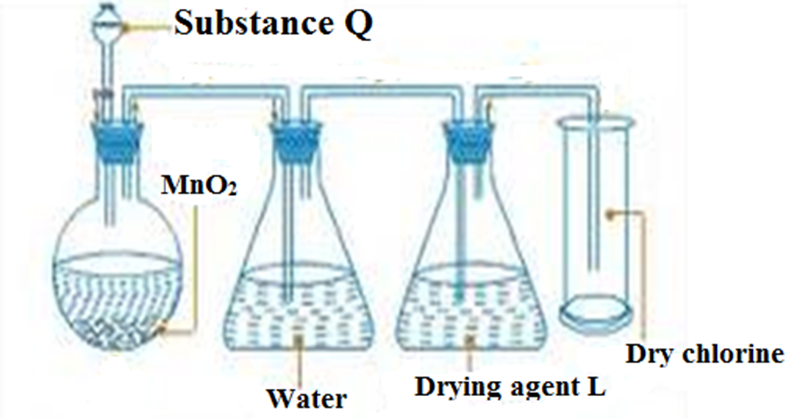
a)Name:
(i) Substance Q
Concentrated hydrochloric acid
(ii) Suitable drying agent L
-Concentrated sulphuric (VI) acid
-anhydrous calcium chloride
-silica gel
b) State a missing condition for the reaction to take place faster.
-Heat/Heating
c) Red and blue litmus papers were dipped into the chlorine gas from the above set up .State and explain the observations made.
Observation: Blue litmus paper remains blue. Red litmus paper remain red.
Explanation: Dry chlorine has no effect on dry litmus papers.
d) Write the equation for the reaction taking place in the conical flask
MnO4 (s) + 4HCl(aq) -> MnCl2(aq) + 2H2O(l) + Cl2(g)
e) Name two other substances that can be used in place of MnO2
Lead(IV) oxide (PbO2)
Potassium manganate(VI)(KMnO4)
Potassium dichromate(K2Cr2O4)
Bleaching powder(CaOCl2)
7. The set up below shows the apparatus used to prepare and collect anhydrous iron(III) chloride.
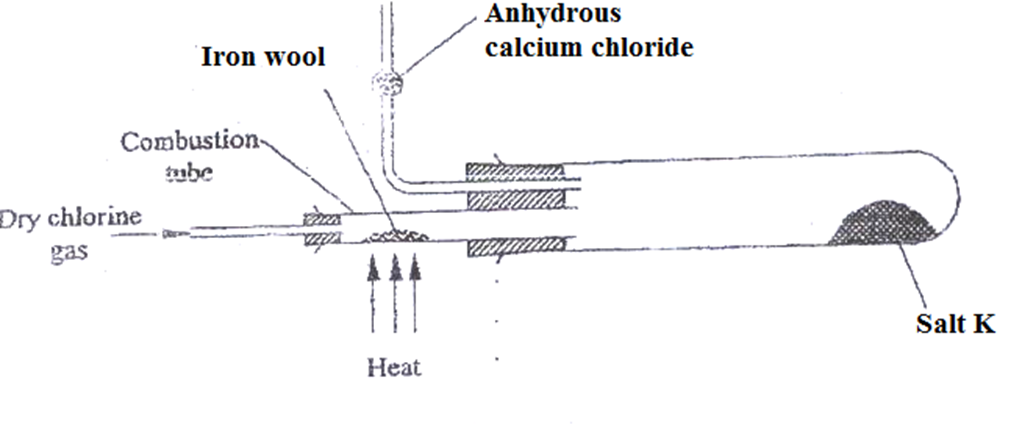
a)Name salt K
Iron(III)cchloride
- Write the equation for the reaction for the formation of salt K
2Fe(s) + 3Cl2 (g) -> 2FeCl3 (s/g)
State and explain the following
(i)Small amount of water is added to iron (II) chloride in a test tube then shaken
Solid dissolves to form a green solution. Iron(II) chloride is soluble in water
(ii)I.Three drops of aqueous sodium hydroxide is added to aqueous iron(II) chloride and then added excess of the alkali.
Observation:
Green precipitate is formed that persist/remain /insoluble in excess akali.
Explanation:
Iron(II) chloride reacts with aqueous sodium hydroxide to form a green precipitate of iron(II) hydroxide.
Ionic equation:
Fe2+(aq) + OH–(aq) -> Fe(OH)2(s)
II.Six drops of hydrogen peroxide is added to the mixture in d(ii) above.
Observation:
Effervescence/bubbling/fizzing take place and the green precipitate dissolve to form a yellow/brown solution.
Explanation:
hydrogen peroxide oxidizes green Fe2+to yellow/ brown Fe3+solution.
9.Use the flow chart below to answer the questions that follow.
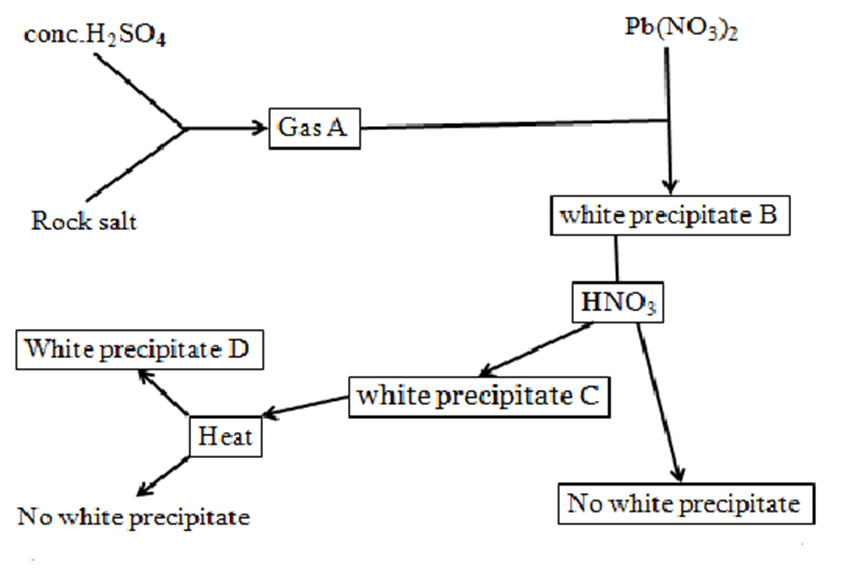
a) Write the chemical equation for the formation of gas A
NaCl(s) + H2SO4(l) -> NaHSO4(aq) + HCl(g)
b) Identify:
(i) four possible ions that can produce white precipitate B
SO42-,SO32-, CO32-, Cl–
(ii)two possible ions that can produce;
I.White precipitate C
SO42-,Cl–
II.colourless solution D
SO32-, CO32-
(iii)possible ions present in
I.White precipitate E
SO42-
II.colourless solution F
Cl–
11. Below is a set up in the preparation of a particular salt. Study it and answer the questions that follow.
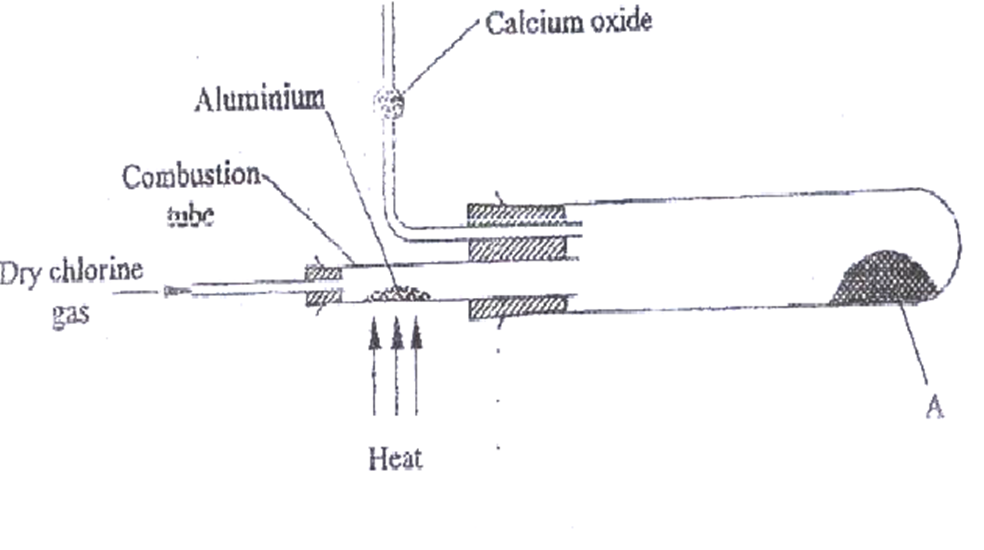
State the observation made when aluminium wool is heated.
Glows red hot.
b)(i) Identify salt A
aluminium(III) chloride// AlCl3
(ii)Write the equation for the formation of salt A
2Al(s) + 3Cl2(g) -> 2AlCl3(s/g)
(iii)What property of salt A is exhibited as shown in the experiment.
It sublimes//sublimation.
(iv)Calculate the minimum volume of chlorine required to form 700kg of iron(III) chloride at room temperature.(Fe= 56.0, Cl=35.5, 1 mole of a gas =24000cm3, 1000g = 1kg)
Mole ratio Fe : Cl2 = 2: 3 molar mass FeCl3 = 162.5g
Method 1
2 x 162.5 g FeCl3 -> 3x 22400 cm3 Cl2
700 x1000 gFeCl3 -> (700 x1000 x3 x22400)/(2 x 162.5)
=1.4474 x 10-8 cm3
Method 2
Moles of FeCl3= mass/ molar mass
=> (700 x 1000) / 162.5 = 4307.6923 moles
Moles of Cl2= 3/2 moles of FeCl3
=>3/2 x 4307.6923 = 6461.5385 moles
Volume of chlorine= moles x molar gas volume
=>6461.5385 x 24000 = 1.5508 x 10-8 cm3
c) Name another metal that can produce similar results as salt K.
Iron
d)(i) What is the purpose of anhydrous calcium chloride.
-ensure the apparatus are water free.
-prevent water from the atmosphere from entering and altering//hydrolysing salt A
(ii) Write the equation for the reaction that take place if anhydrous calcium chloride is not used in the above set up.
AlCl3(s) + 3H2O(l) -> Al(OH)3(aq) + 3HCl(g)
(iii) Write the equation for the reaction that take place when Iron metal is reacted with dry hydrogen chloride gas.
Fe(s) + 2HCl(g) -> FeCl2(s) + H2(g)
(iv)Calculate the mass of Iron(II)chloride formed when 60cm3 of hydrogen chloride at r.t.p is completely reacted. (1 mole of a gas =24dm3 at r.t.p, Fe = 56.O, Cl= 35.5)
Chemical equation Fe(s) + 2HCl(g) -> FeCl2(s) + Cl2(g)
Mole ratio HCl: FeCl2 = 1:1
Molar mass FeCl2 = 127g
Moles of HCl used = 60cm3 /24000cm3 = 2.5 x 10 -3 moles
Moles of FeCl2 = Moles of HCl => 2.5 x 10 -3 moles
Mass of FeCl2 = moles x molar mass => 2.5 x 10 -3 x 127 =0.3175g
12.Study the flow chart below and use it to answer the questions that follow
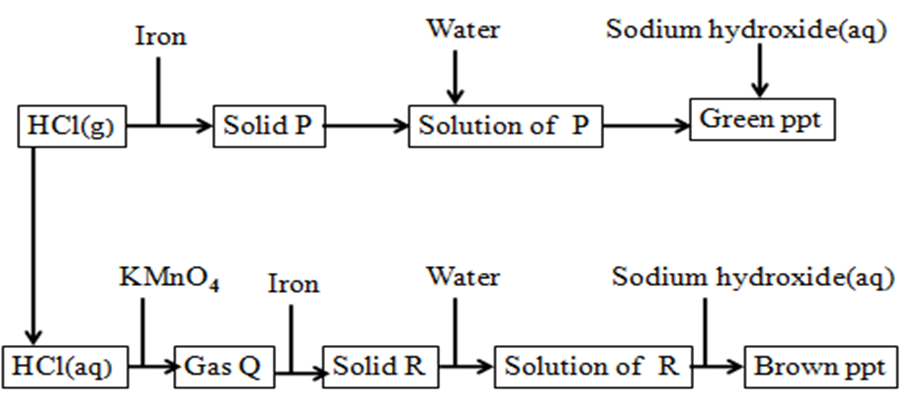
a)Identify substance:
P Iron(II) chloride//FeCl2
Q Chlorine // Cl2
R Iron(III) chloride//FeCl3
b)Write the equation for the reaction for the formation of:
(i) gas Q
2KMnO4 (s) + 16HCl(aq) -> 2KCl(aq) + 2MnCl2(aq) + 8H2O(l) + 5Cl2(g)
(ii) the green precipitate (using ionic equation)
Ionic equation:
Fe2+(aq) + 2OH–(aq) -> Fe(OH)2(s)
(ii) the brown precipitate (using ionic equation)
Ionic equation:
Fe3+(aq) + 3OH–(aq) -> Fe(OH)3(s)
c)A glass rod was dipped in aqueous ammonia. The rod was then brought near hydrogen chloride. State and explain the observation made.
Observation:
White fumes
Explanation:
Ammonia gas reacts with hydrogen chloride gas to form white fumes of ammonium chloride.
Chemical equation:
NH3(g) + HCl(g) -> NH4Cl(s)
13. Using dot(.)and cross(x)to represent electrons,show the bonding in aluminium chloride in vapour phase.
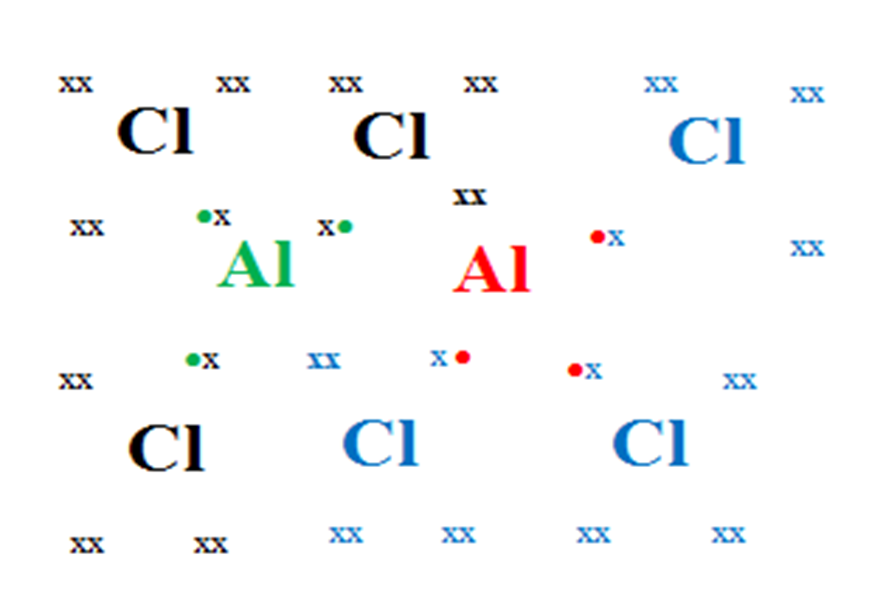
(b)How many electrons in :
(i)aluminium atoms are used in bonding.
Six electrons(three valence electrons in each aluminium atom)
(ii)chlorine atoms atoms are used in dativebonding.
four electrons(two lone pairs of valence electrons in two chlorine atoms)
(iii)the molecule are used in bonding.
Sixteen electrons
-six valence electrons from aluminium atom through covalent bond
-six valence electrons from chlorine atoms through covalent bond.
– four valence electrons from chlorine atoms through dative bond
(c)How many lone pair of electrons do not take part in bonding within the molecule.
Sixteen(16) lone pairs from six chlorine atoms(32 electrons)
(d)Aluminium chloride does not conduct electricity in molten state but Magnesium chloride conduct.
Aluminium chloride is a molecular compound that has no free mobile Al3+ and Cl– ions which are responsible for conducting electricity. Magnesium chloride has free mobile Mg2+ and Cl– ions because it is an ionic compound.
8. Use the flow chart below to answer the questions that follow:
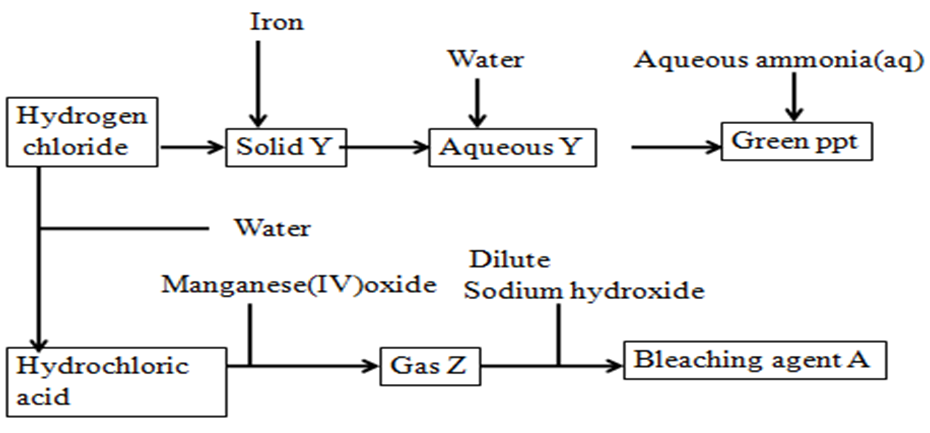
a)Write an equation for the school laboratory formation of hydrogen chloride gas
NaCl(s) + H2SO4(l) -> NaHSO4(aq) + HCl(g)
KCl(s) + H2SO4(l) -> KHSO4(aq) + HCl(g)
b)Name:
I. solid Y Iron (II) chloride (FeCl2)
II green precipitateIron (II) hydroxide (Fe (OH)2
III Gas Y Chlorine (Cl2)
IV. Bleaching agent A Sodium hypochlorite (NaOCl)
c)Blue and red litmus papers were dipped into bleaching agent A. Write the equation for the reaction that takes place.
Coloured dye +NaOCl(aq) ->NaCl(aq) + (Colourless dye + O)//
(Coloured dye-O) + NaOCl(aq) ->NaCl(aq) + Colourless dye
d)State four uses of gas Z
- Bleaching agent
- Manufacture of hydrochloric acid
- Chlorination of water to kill germs
- Manufacture of PVC pipes
