Chemistry Form 3 Past Paper Questions with Marking Scheme
1. State two reasons why luminous flame is not used for heating purposes in the laboratory(2mks)
| . |
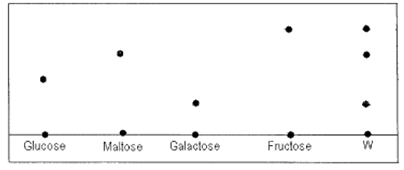
(a) Name the sugar present in W (2mks)
(b) Which of the sugars has the highest density? Explain. (2mks)
3. A student was asked to determine the percentage of zinc metal in a mixture of zinc metal and zinc oxide. He reacted the mixture with excess hydrochloric acid and accurately collected the gas evolved, which was then used to calculate the amount of zinc in the mixture.
(a) Name the gas that was evolved? (1mk)
(b) Apart from the reaction liberating the gas write a balanced equation for the other reaction that took place . (1mk)
(c) Why would dilute nitric acid not suitable for this reaction? (1mk)
4 a. (i) Complete the table below (2mks)
| Species | Number of neutrons | Number of electrons |
| 3 He2+ 2 |
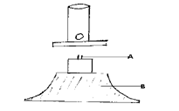
5. Filtration is carried out in the apparatus shown
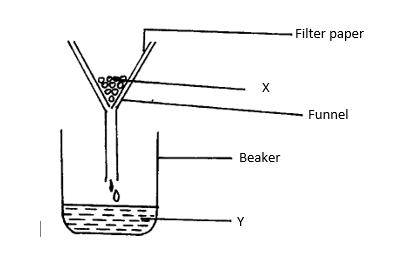
(1mk)
Name X and Y
X: .
Y: .
6. a) State Charles’ law: (1mk)
b) A gas R at 27°C and 750mmHg was found to occupy 36cm3. calculate the temperature at which the same mass of R will occupy twice the volume at a pressure of 1000mmHg (3mks)
7. The data below gives the electronic configuration of some selected atoms and ions
| Atom/ion | A2+ | B | C2- | D2+ | E | F– | G+ | H |
| Electronic configuration | 2 | 2.4 | 2.8 | 2.8.8 | 2.8 | 2.8.8 | 0 | 2.8.2 |
(a) Select an atom that is a noble gas (1mk)
(b) What is the atomic number of C and A (2mks)
(c) Select an element that belong to group 2 and period four (1mk)
(d) Write the formula of the compound formed when D and F react (1mk)
.
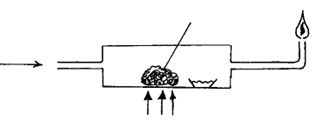
8. The diagram below shows a setup used to burn charcoal. Study it and answer the questions that follow.
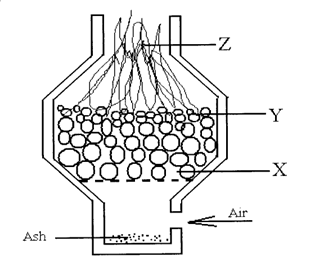
a) Write an equation for the reaction that occurs at point Y (1 mk)
b) Explain using equations what happens at point Z. (1 mk)
c) State one precaution to be taken when using charcoal stoves in our homes (1 mk)
9. The set up below was used to prepare a sample of oxygen gas. Study it and answer the questions that follow.
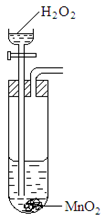
(i) Complete the diagram to show how Oxygen can be collected. (2mks)
(ii) Write a chemical equation of the reaction that produced oxygen. (1mk)
10. Element A and B with atomic numbers 12 and 17 respectively react together.
a. Write the electronic configurations of each (2mks)
A:
B:
b. Write the formula of the compound formed between A and B. (1mk)
11. Matter exists in three states which can be related as shown in the diagram below.
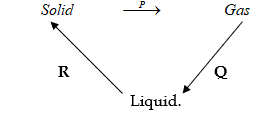
(a) Name processes: P: (1mk)
R: . (1mk)
(b) Give two examples of substances that undergo process P (1mk)
12. The structure of water molecule can be represented as shown below.
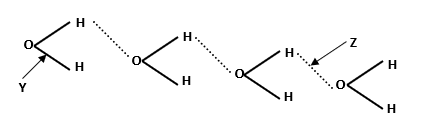
a) Name the bond type represented by letters Y and Z
Y: . (1mk) Z: . (1mk)
b) Methane and water are molecular substances with almost similar molecular masses however; the boiling point of water is 1000C while that of methane is – 1610C. Explain (1mk)
13. Study the diagram below and answer the questions that follow.
a) State two observations made during the above experiment. (2mks)
b) Write down the equation for the reaction taking place in the combustion tube. (1mk)
c) Give a reason why excess gas is burnt. (1mk)
14. A certain element A whose atomic number is 14 has 3 isotopes. The table below shows the mass number and relative abundance of each isotope.
| Isotopic mass | % abundance |
| 28.0 | 92.2 |
| 29.0 | 4.7 |
| 30.0 | 3.1 |
Calculate the relative atomic mass of element A. (3mks)
15. Some potassium chloride was found to be contaminated with copper (II) oxide. Describe how a sample of potassium chloride can be obtained from a mixture. (3mks)
16. Study the diagram below and answer the questions that follow. The diagram shows the method of separating components of mixture Q.
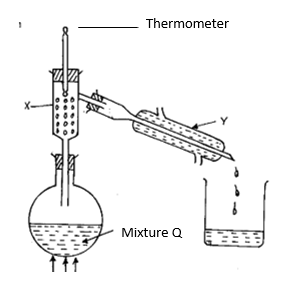
Heat
a) Name apparatus X and Y. (1mk)
X: .
Y: .
b) What is the purpose of apparatus X? (1mk)
c) Show the direction of flow of cold water used for cooling the vapour formed. (1mk)
d) What name is given to the above method of separating mixtures? (1mk)
17. (a) The diagram below shows some parts of a Bunsen burner. Study it and answer the
questions that follow
Explain how the parts labelled A and B are suited to their functions
a. A:. (1mk)
b. B: (1mk)
(b) State two reasons why most apparatus in the laboratory are made of glass (2mk)
18. The table below gives some properties of elements X, Y and Z in their solid state. Study it and answer the question that follows.
| Solid | Solubility in ethanol | Attraction by magnet |
| X | Insoluble | Not attracted |
| Y | Insoluble | Attracted |
| Z | Soluble | Not attracted |
Explain how a pure sample of Z can be obtained from a mixture of X and Z (3mks)
19. The figure below shows some steps in the industrial preparation of sodium carbonate. Study it and answer the questions that follow.
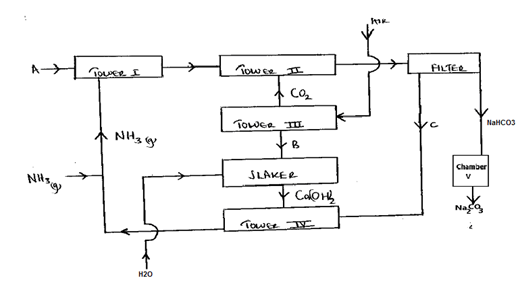
i. Identify substances labelled A and B (2mks)
ii. Cold water is made to circulate around Tower II. Explain (1mk)
.
iii. Name the method that is used to separate sodium hydrogen carbonate and ammonium chloride (1mk)
iv. Name one substance used in Tower III to produce Carbon (IV) oxide (1mk)
v. Write the equation for the conversion of sodium hydrogen carbonate to sodium carbonate stating the condition for the reaction (2mks)
.
20. The following apparatus is commonly used in a chemical laboratory. Give its name and state its use.
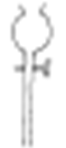
(2mks)
Name
Use
21. In an experiment to determine the percentage of impurity in Sodium carbonate, 1.8g of impure Sodium carbonate was reacted with excess 2M Hydrochloric acid. 340cm3 of dry Carbon (IV) oxide gas was collected during the experiment at room temperature and pressure.
(Na=23, 0=16, C=12; Molar gas volume at r.t.p=24dm3)
a) Why was excess 2M Hydrochloric acid used in the experiment? (1mk)
b) Write an equation for the reaction that produced Carbon (IV) oxide (1mk)
c) Calculate
i. The number of moles of Carbon (IV) oxide produced (2mks)
ii. The number of moles of Sodium carbonate that reacted with the acid (2mks)
iii. The mass of Sodium carbonate that reacted with the acid (2mks)
iv. The percentage of impurities in the sample of Sodium carbonate (2mks)
22. a) What is meant by allotropy? (1mk) .
b) The diagram below shows the structure of one of the allotropes of carbon
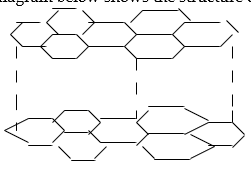
a. Identify the allotrope (1mk)
b. State one property of the above allotrope and explain how it is related to its structure.(2ms)
 Join Our WhatsApp Group
Join Our WhatsApp Group