CHEMISTRY FORM 1
END OF TERM 3 EXAM – 2022 TIME: 2HRS
INSTRUCTIONS
- ANSWER ALL THE QUESTIONS IN SPACES PROVIDED
- The pH scale has a range of values ranging from 0 to 14. On the pH scale, any substance with a pH value of 7 is neutral. Classify the following substances as either strong or weak acids or bases using their pH values. (4mks)
(a) pH 8
(b) pH 6
(c) pH1
(d) pH12
2. Grape juice is sour while aloe juice tastes bitter. What name is given to substances which taste (2mks)
(a) Sour
(b) Bitter
3. Complete the following word equation. (7mks)
(a) Acid + base …………… + ……………………
(b) Acid + ………………. salt + hydrogen gas
(c) Acid + Carbonate …………………..+ ……………………………. + water
(d) Acid + Hydrogen Carbonate salt + ……………………..+………………………
4. Study the table and answer the questions that follow. (3mks)
| Solution | Indicators | ||
| Colour in A | Colour in B | Colour in C | |
| Soap | Purple | Colourless | Orange |
| Baking powder | Blue | Pink | Yellow |
| Dilute hydrochloric acid | RED | Colourless | Pink |
| Vinegar | Red | Colourless | Pink |
Identify the indicators A, B, and C
A
B
C
5. Use the diagram below to answer the questions that follow:
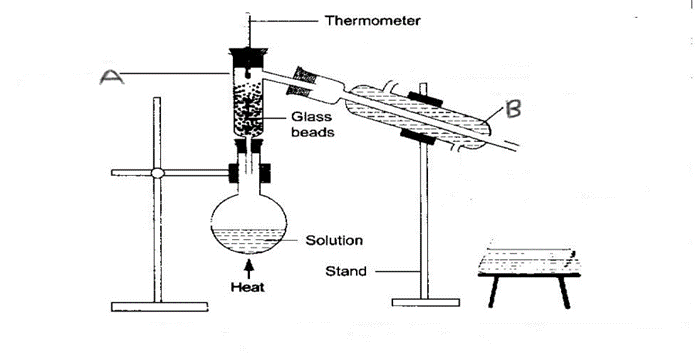
(a) Name the process of separation above. (1mk)
(b) Name the parts labeled A and B. (2mks)
A
B
(c) Explain the role of glass beads. (1mk)
(d) Indicate the direction of flow of water in apparatus B. (2mks)
5. Matter may change from one state to another under sustain conditions. Study the illustration below and answer question that follow. (6mks)
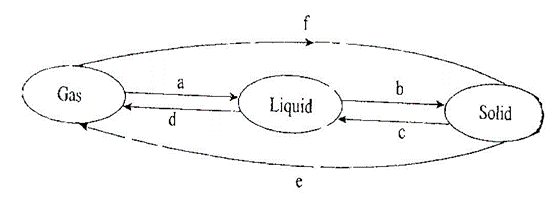
Name process A to F
A
B
C
D
E
F
6. Consider the diagram below. (3mks)
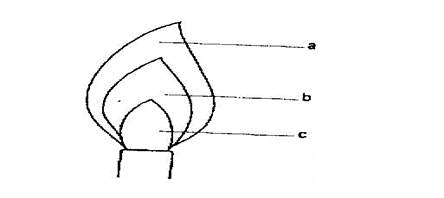
Name the parts indicated on the diagram which are represent as a,b,c,
(i) Unburned gas zone
(ii) Pale blue zone
(iii) Green blue zone
7. State any five laboratory safety rules. (5mks)
8. (a) The following table contains some elements. Study them and complete the table with correct chemical symbols. (5mks)
| Elements | Chemical symbol |
| Sulphur | |
| Carbon | |
| Oxygen | |
| Hydrogen | |
| Nitrogen | |
| Magnesium | |
| Calcium | |
| Sodium | |
| Chlorine | |
| Copper |
(b) The following table contains chemical symbols. Write down the name of the element represented by the symbol. (5mks)
| Chemical symbol | Name |
| Pb | |
| Fe | |
| K | |
| Ag | |
| Hg |
- 9. What is matter? (1mk)
- 10. Name the three states of matter (3mks)
- 11. The diagram below shows chromatograms for five different dyes.
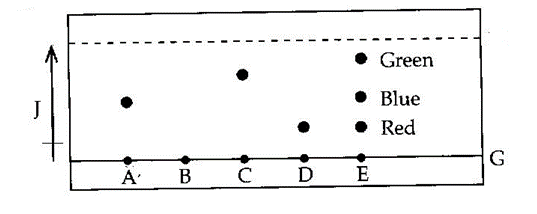
(a) Name the technique used to separate the dyes. (1mk)
(b) What properties are required to separate the chromatograms in a dye? (1mk)
(c) On the diagram above label the solvent front by using a letter H. (1mk)
(d) Which letter represent baseline on the diagram. (1mk)
(e) Which dye is insoluble? (1mk)
(f) Which dye is pure? Explain (1mk)
(g) Which chromatogram is most soluble? (2mks)
12. Give five differences between non-permanent (physical) and permanent (chemical). (5mks)
Non – permanent | Permanent |
- 13. (a) Study the following table and classify the changes as non-permanent (physical) or permanent (chemical) after the action of heat. (2mks)
| Substance | Original colour before heating | Colour of residue after heating | Type of change | |
| 1 | Copper ii Sulphate | Blue crystals | White powder (solid) | |
| 2 | Potassium | Purple | Black solid | |
| 3 | Zinc oxide | White (oxide) | Yellow when hot and white when cold | |
| 4 | Iodine | Black solid | Purple vapour when hot and black solid when cold |
When water was added to the white Copper II Sulphate it turned to blue, explain the type of change. (1mk)
(i) What name is given to the blue copper II Sulphate? (1mk)
(ii) White Copper II Sulphate. (1mk)
- What is the confirmatory test for the presence of pure water? (1mk)
- Define the following
(i) Mixture (1mk)
(ii) Compound (1mk)
(iii) Write a word equation when iron metal is heated together with Sulphur element to form iron II Sulphide. (1mk)
- Name any four apparatus used to measure accurate volumes in the laboratory. (4mks)
