Chemistry Form 4 Past Paper 2 Questions with Marking Scheme
1. (a) Study the flow chart below and answer the questions that follow.
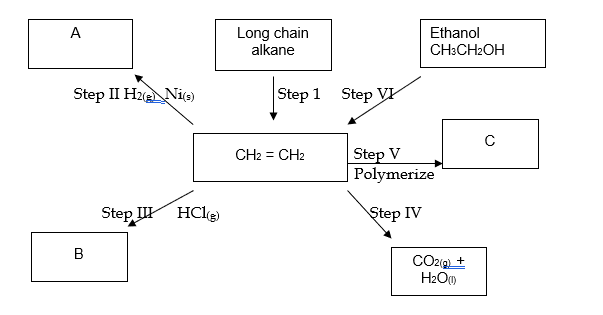
(i) Name the process taking place in step (I). (1mark)
(ii) Describe a chemical test that can be carried out to show the identity of organic compound A.
(2marks)
(iii) Give the name of the following: (2marks)
I. A:
II. B:
(iv) Give the structural formulae of substance C. (1mark)
.
(v) Name the type of reaction that occurs in:
I. Step IV (2marks)
II. Step VI:
(vi) Give the reagent and the condition necessary for step VI. (2marks)
Reagent:
Condition:
(b) Give the systematic names of the following compounds:
I. CH2CHCHCH2CH3 (1mark)
II. CH C CH3 (1mark)
2. a) The results below were obtained in an experiment conducted by form 3 students from
Tigityo Secondary school using Magnesium.
– Mass of the crucible + lid = 19.52g
– Mass of the crucible + lid + Magnesium Ribbon = 20.36g
– Mass of the crucible + lid + Magnesium oxide = 20.92g
(i) Use the results to find the percentage mass of Magnesium & Oxygen in Magnesium oxide
(2 marks)
(ii) Determine the empirical formula of magnesium oxide. (Mg=24.0,O=16.0) (3 marks)
.
b) Sodium hydroxide pellets were accidentally mixed with sodium chloride 8.8g of the
mixture were dissolved in water to make one litre of solution. 50cm3 of the solution
was neutralised by 20cm3 of 0.25M sulphuric acid.
(i) Write an equation for the reaction that took place. (1 mark)
(ii) Calculate the:
I. number of moles of the substance that reacted with sulphuric acid. (2 marks)
II. number of moles of the substance that would react with sulphuric acid in the one litre
solution (2 marks)
(iii) the percentage of sodium chloride in the mixture. (2 marks)
(H=1.0; Na=23.0; Cl=35.5; O=16.0)
3. a) Study the table below and answer the questions that follow
Bond type bond energy kJmol-1
C-C 346
C = C 610
C-H 413
C-Br 280
Br-Br 193
i) Calculate the enthalpy change for the following reaction (3 marks)
C2H4(g) + Br2(g) C2H4Br2(g)
ii) Name the type of reaction that took place in (a) above (1mark)
b) Butane C4H10 cannot be prepared directly from its elements but its standard heat of formation () can be obtained indirectly.
The following heats of combustion are given.
(Carbon) = -393kJ/mol
(Hydrogen) = -286kJ/mol
(Butane) =-2877kJ/mol
i) Draw an energy cycle diagram linking the heat of formation of butane with its heat of combustion and the heat of combustion of its constituents elements. (1mark)
ii) Calculate the heat of formation of butane (C4H10) (2marks)
c) Given that the lattice enthalpy of potassium chloride is +690kJ/mol and hydration enthalpies of K+ and Cl– are -322kJ and -364kJ respectively. Calculate the enthalpy of solution of potassium chloride. (3 marks)
4. (a) Name two apparatuses that can be used for determining accurate volume in a
laboratory (2marks)
(b) One of the flames produced by Bunsen burner is the luminous flame
i) Explain why this flame is very bright (1mark )
ii) State two disadvantages of the luminous flame (2marks)
(c) Air is usually one of the substances that is considered as a mixture
(i) Identify the two most abundant component of air (2marks)
(ii) Give two reasons why the air is considered as a mixture (2marks)
(iii) One of the components of air is carbon (iv) oxide. Describe an experiment that can be used to prove the presence of carbon (iv) oxide in the air (2marks)
5. The grid below forms part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements
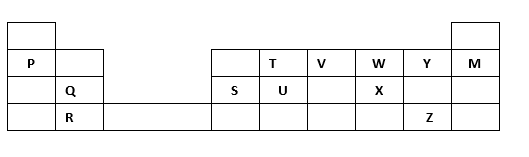
a) Write the general name given to the element P belong. (1mark)
b) An element N has an atomic number of 15. Write down its electronic arrangement and hence fix it in its right position on the grid above. (2marks)
Electronic arrangement
c) Compare the size of the atom of R and that of its ion. Explain your answer. (2marks)
d) Give the formula of the compound formed between (1mark)
i. P and W
ii. T and Y
e) Compare the melting points of element Q and S. Explain (2marks)
f) State the least reactive element in the grid. Give a reason for your answer (2marks)
g) Give two advantages that element S has over element Q in making electric cables(2mks)
h) Draw (a) dot (.) and cross (x) diagram to represent the bonding in compound formed between T and Y (2 marks)
6. The chart below represents the main steps in the large-scale manufacture of sodium carbonate.
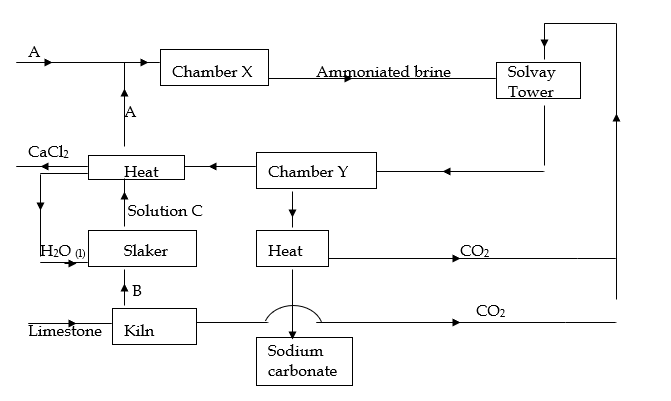
(a) Name substances A and B.
A (1 mark )
B ( 1 mark)
(b) Write down the chemical equation leading to formation of C. (1 mark )
(c ) A stream of cold water is made to circulate around chamber X. What does this
suggest about the reaction taking place. (1 mark )
(d) Name the process that takes place in chamber Y. (1 mark)
(e) State any 2 by-products recycled in the process. (2 marks)
(f) In an experiment, wood charcoal was mixed with concentrated sulhuric (VI) acid in
a test-tube. The mixture was then placed over a Bunsen-burner flame for sometime.
(i) Write down the chemical equation of the reaction that takes place. (1 mark)
.
(ii) State the property of concentrated sulphuric (VI) acid investigated in (i) above. (1 mark)
(g) Mention any 2 uses of sodium carbonate. ( 1 mark )
7. The set-up below can be used to generate a gas.
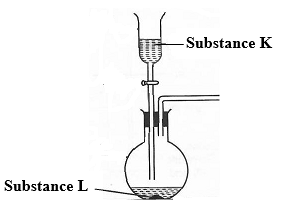
(a) (i) Complete the table below giving the names of substance K and L if the gases generated
are carbon (IV) oxide and carbon (II) oxide. (2marks)
| Substance | Carbon (IV) oxide | Carbon (II) oxide |
| K | ||
| L |
(ii) Complete the diagram to show how a sample of carbon (II) oxide can be collected (2marks)
(iii) State two ways that can be used to distinguish carbon (IV) oxide from carbon (II)
oxide? (2 marks)
(b) (i) In an experiment, carbon (IV) oxide gas was passed over heated charcoal held in a
combustion tube. Write a chemical equation for the reaction that took place in the
combustion tube. (1 mark)
(ii) State one use of carbon (II) oxide. (1 mark)
(c) The following set ups were used by Form Two students. Study and use them to answer the
questions that follow.
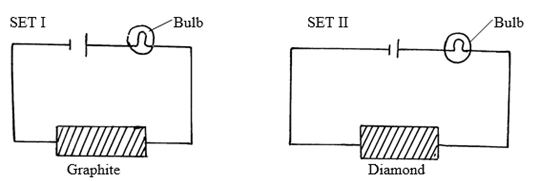
State and explain the difference in observation made in set up I and II above. (3 marks)
 Join Our WhatsApp Group
Join Our WhatsApp Group