Chemistry Form 2 Past Paper Questions with Marking Scheme
1. a) Distinguish between atomic number and mass number (2mks)
b) State three sub-atomic particles in an atom (3mks)
2. The element sodium, lithium and potassium form an important family of element
(a) Name the family in which these elements belong (2mks)
(b) State and explain the trends with reference to the above family in properties down the group
(i) Atomic radius (2mks) ..
(ii) Ionic radius (2mks)
(c) How does the ionic radius and atomic radius of a given element in a family compare (1mk)
3. An element whose atomic is 14 has three isotopes A,B and C. use the information given in the table below to answer the question that follow
| Isotope | Mass number | % abundance |
| A | 28 | 92.0 |
| B | 29 | – |
| C | 30 | 3.0 |
a) Determine the relative abundance of isotope B (1mk)
b) Calculate the relative atomic mass of element B above (3mks)
4. A sample of water in a beaker was found to boil at 101.5oC at 1 atmospheric pressure. Assuming that the thermometer was not faulty, explain the observation (3mks)
5. A student set up the test tubes to investigate rusting of iron. Study it and answer the question that follows.
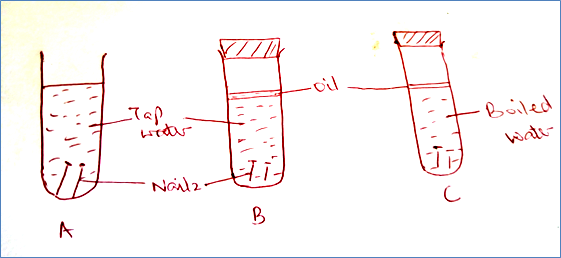
a) Identify the tubes where rusting occurred (1mk)
.
b) With a reason state the test tube where rusting did not occur (2mks)
6. A mixture was found to contain sodium chloride, sand and iodine. Describe how each of the substances can be obtained from the mixture (3mks)
7. The diagram below represents part of the periodic table. Use it to answer the question that follows
| A D B C E F |
a) Write electronic arrangement of the following element (3mks)
A
D.. E
b) Write the formula of the compound found between (2mks)
i) C and D
ii) E and D
.
8. (a) State two types of flames and in each explain how it can be produce (2mks)
(b) State two ways of preventing rusting (2mks)
9. The table below gives the number of electrons, protons and neutrons in substances X,Y and Z. Study it and answer the question that follows.
| Substance | Electrons | Protons | Neutrons |
| X | 10 | 10 | 10 |
| Y | 10 | 8 | 10 |
| Z | 8 | 8 | 8 |
i) Which letter represents an ion (1mk)
ii) Which of the substances are isotopes? Give a reason for your answer. (2mks)
iii) Draw the atomic structure of element Z (2mks)
10. The diagram below shows the relationship between the physical states of matter. Study it and answers the questions that follows.
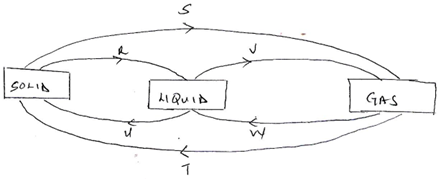
a) Identify the process R,V,W and U (4mks)
b) Name three substances which can undergo the process represented by process S and T. (3mks)
11. The figure below shows a heating curve of a certain pure solid.
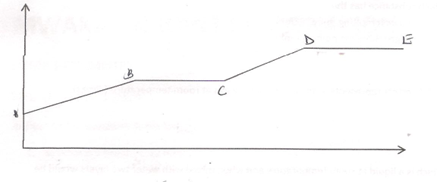
a) What is happening at the stages represented by BC and CD ( 4mks)
.
b) On the diagram draw a heating curve of an impure substance. (2mks)
12.a) Write an equation for the reaction to show how hydrogen gas is prepared in the laboratory
(1mk)
b. State any three uses of hydrogen gas (2mks)
13. State three apparatus that are used in the laboratory to accurately measure volume.
(3mks)
14. An impure substance Z was subjected to a chromatographic analysis with other three substances. The following chromatogram was formed. Use it to answer the question that follows.
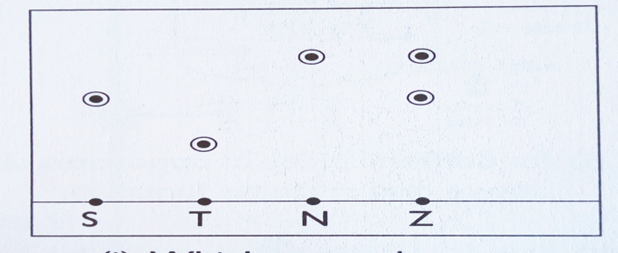
a) Which substances make up Z (2mks)
b) i) Which component of Z is more soluble in the solvent used (1mk)
ii) Explain your answer in b(i) above (2mk)
……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………..
c) Indicate the solvent front on the chromatogram (1mk)
15. State two reasons why luminous flame is not used for heating purposes in the laboratory
(2mks)
| . |
16. Complete the table below (2mks)
| Species | Number of neutrons | Number of electrons |
| 27 Al3+ 13 |
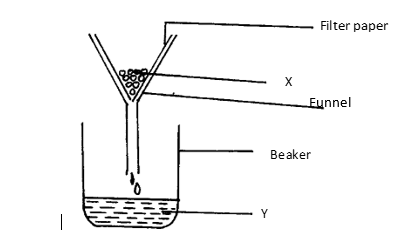
17. Filtration is carried out in the apparatus shown
(2mks)
Name X and Y
X: .
Y: .
18. The set up below was used to prepare a sample of oxygen gas. Study it and answer the questions that follow.
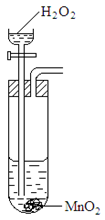
(i) Complete the diagram to show how Oxygen can be collected. (2mks)
19. Classify the following processes as either chemical or physical (3mks)
| Process | Type of Change |
| i. Rusting of Iron | |
| ii. Souring of Milk | |
| iii. Melting of ice |
20. Write the formula of the following compounds (3mks)
a) Sodium nitrate: ..
b) Potasium Sulphate: …
c) Calcium Carbonate: .
21. Balance the following Chemical equations (2mks)
a) Mg(s) + HCl (aq) MgCl2(aq) + H2(g)
b) Na2CO3(s) + HNO3(aq) NaNO3(aq) + CO2(g) + H2O(l)
 Join Our WhatsApp Group
Join Our WhatsApp Group