Introduction to Chemistry Practicals /Rationale
Chemistry is a science.
Chemistry practical all over the world is emphasized to all candidates sitting for a Chemistry paper.
There are about seven main basic universal emphasis for all chemistry candidates sitting for a chemistry paper;
(i)Titration /volumetric analysis
(ii)Thermochemistry(energy changes)
(iii)Chemical kinetic(rates of reaction)
(iv)Qualitative analysis(organic/inorganic)
(v)Solubility and solubility curves
(vi)Flame test
(vii)Physical / general chemistry
Chemistry Practicals: Titration / Volumetric Analysis
Titration is determining the end point of the burette contents that react with fixed (usually 25.0cm3 from a pipette) conical flask contents.
As evidence of a titration actually done examining body require the candidate to record their burette readings before and after the titration.
For KCSE candidates burette readings must be recorded in a titration table in the format provided by the Kenya National Examination Council.
As evidence of all titration actually done Kenya National Examination Council require the candidate to record their burette readings before and after the titration to complete the titration table in the format provided.
Sample Titration table format
| Final burette reading (cm3) | 24.0 | 24.0 | 24.0 |
| Initial burette reading (cm3 | 0.0 | 0.0 | 0.0 |
| Volume of solution used(cm3) | 24.0 | 24.0 | 24.0 |
Calculate the average volume of solution used
24.0 + 24.0 + 24.0 = 24.0 cm3
3
As evidence of understanding the degree of accuracy of burettes ,all readings must be recorded to a decimal point.
As evidence of accuracy in carrying the out the titration ,candidates value should be within 0.2 of the school value .
The school value is the teachers readings presented to the examining body/council based on the concentrations of the solutions s/he presented to her/his candidates.
Bonus mark is awarded for averaged reading within 0.1 school value as Final answer.
Calculations involved after the titration require candidates thorough practice mastery on the:
(i)relationship among the mole, molar mass, mole ratios, concentration, molarity.
(ii) mathematical application of 1st principles.
Very useful information which candidates forget appear usually in the beginning of the paper as:
“You are provided with…”
All calculation must be to the 4th decimal point unless they divide fully to a lesser decimal point.
Never round off answers.
Practicals in Thermochemistry / Energy Changes
Energy is the capacity to do work which is measured in Joules(J) or(kJ) .
Chemical/physical changes take place with absorption (Endothermic ) or evolution/ production (Exothermic)of heat.
Practically:
(i)endothermic changes show absorption of heat by a fall / drop in temperature and has a +∆H
(ii)exothermic changes show evolution/ production of heat by a rise in temperature and has a -∆H
(iii)temperature is measure using a thermometer.
(iv)a school thermometer is either coloured (alcohol) or colourless(mercury)
(v) For accuracy ,candidates in the same practical session should use the same type of thermometer.
(vi) fall / drop (+∆H) in temperature is movement of thermometer level downward.
(vii) rise (-∆H) in temperature is movement of thermometer level upwards.
Physical changes changes mainly involve melting/freezing/fussion and boiling /vapourization.
Chemical changes changes mainly involve displacement ,dissolving , neutralization
a).Energy changes in physical processes
Melting/freezing/fusion/solidification and boiling/vaporization/evaporation are the two physical processes.
Melting /freezing point of pure substances is fixed /constant.
The boiling point of pure substance depends on external atmospheric pressure.
Melting/fusion is the physical change of a solid to liquid. Freezing/fusion is the physical change of a liquid to solid.
Melting/freezing/fusion/solidification are therefore two opposite but same reversible physical processes. i.e
A (s) ========A(l)
Boiling/vaporization/evaporation is the physical change of a liquid to gas/vapour. Condensation/liquidification is the physical change of gas/vapour to liquid. Boiling/vaporization/evaporation and condensation/liquidification are therefore two opposite but same reversible physical processes. i.e
B (l) ========B(g)
Practically
- Melting/liquidification/fusion involves heating a solid to weaken the strong bonds holding the solid particles together.
Solids are made up of very strong bonds holding the particles very close to each other (Kinetic Theory of matter
On heating these particles gain energy/heat from the surrounding heat source to form a liquid with weaker bonds holding the particles close together but with some degree of freedom.
Melting/fusion is an endothermic (+∆H)process that require/absorb energy from the surrounding.
(ii)Freezing/fusion/solidification involves cooling a a liquid to reform /rejoin the very strong bonds to hold the particles very close to each other as solid and thus lose their degree of freedom (Kinetic Theory of matter).
Freezing /fusion / solidification is an exothermic (–∆H)process that require particles holding the liquid together to lose energy to the surrounding.
(iii)Boiling/vaporization/evaporation involves heating a liquid to completely break/free the bonds holding the liquid particles together.
Gaseous particles have high degree of freedom (Kinetic Theory of matter). Boiling /vaporization / evaporation is an endothermic (+∆H) process that require/absorb energy from the surrounding.
(iv)Condensation/liquidification is reverse process of boiling /vaporization / evaporation.
It involves gaseous particles losing energy to the surrounding to form a liquid.It is an exothermic(+∆H) process.
The quantity of energy required to change one mole of a solid to liquid or to form one mole of a solid from liquid at constant temperature is called molar enthalpy/latent heat of fusion. e.g.
H2O(s) -> H2O(l) ∆H = +6.0kJ mole-1 (endothermic process)
H2O(l) -> H2O(s) ∆H = -6.0kJ mole-1 (exothermic process)
The quantity of energy required to change one mole of a liquid to gas/vapour or to form one mole of a liquid from gas/vapour at constant temperature is called molar enthalpy/latent heat of vapourization. e.g.
H2O(l) -> H2O(g) ∆H = +44.0kJ mole-1 (endothermic process)
H2O(g) -> H2O(l) ∆H = -44.0kJ mole-1 (exothermic process)
- To determine the boiling point of water
Procedure:
Measure 20cm3 of tap water into a 50cm3 glass beaker. Determine and record its temperature.Heat the water on a strong Bunsen burner flame and record its temperature after every thirty seconds for four minute
Sample results
| Time (seconds) | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 |
| Temperature(oC) | 25.0 | 45.0 | 85.0 | 95.0 | 96.0 | 96.0 | 96.0 | 97.0 | 98.0 |
Questions
1. Chemistry Practical: Plot a graph of temperature against time(y-axis)
Sketch graph of temperature against time
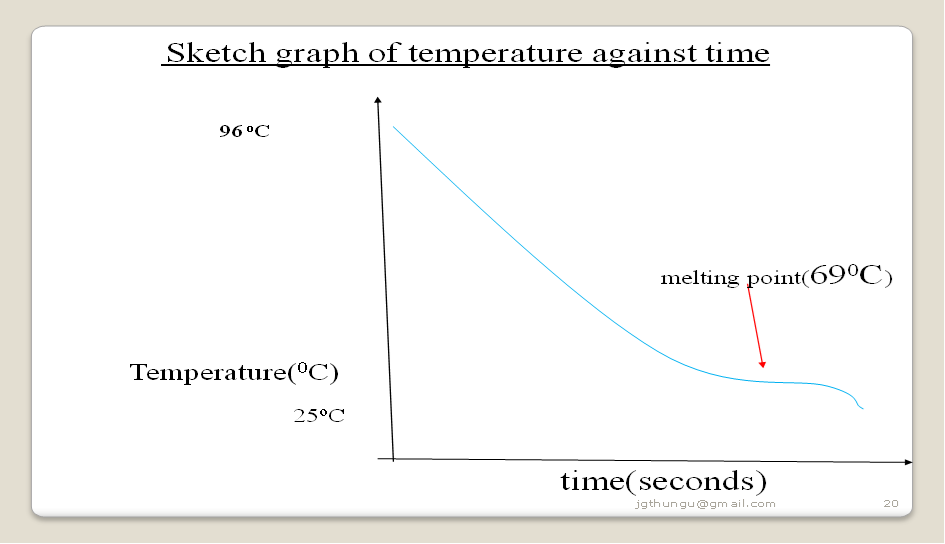
2.From the graph show and determine the boiling point of water
Note:
Water boils at 100oC at sea level/one atmosphere pressure/101300Pa but boils at below 100oC at higher altitudes.
The sample results above are from Kiriari Girls High School-Embu County on the slopes of Mt Kenya in Kenya. Water here boils at 96oC.
3.Calculate the molar heat of vaporization of water.(H= 1.0,O= 16.O)
Working:
Mass of water = density x volume => (20 x 1) /1000 = 0.02kg
Quantity of heat produced
= mass of water x specific heat capacity of water x temperature change
=>0.02kg x 4.2 x ( 96 – 25 ) = -5.964kJ
Heat of vaporization of one mole H2O
= Quantity of heat
Molar mass of H2O
=> -5.964kJ = -0.3313 kJ mole -1
18
To determine the melting point of candle wax
Procedure
Weigh exactly 5.0 g of candle wax into a boiling tube. Heat it on a strongly Bunsen burner flame until it completely melts.
Insert a thermometer and remove the boiling tube from the flame. Stir continuously. Determine and record the temperature after every 30seconds for four minutes.
Sample results
| Time (seconds) | 0 | 30 | 60 | 90 | 120 | 150 | 180 | 210 | 240 | 240 |
| Temperature (oC) | 93.0 | 85.0 | 78.0 | 70.0 | 69.0 | 69.0 | 69.0 | 67.0 | 65.0 | 65.0 |
Questions
1.Plot a graph of temperature against time(y-axis)
b)Energy changes in chemical processes
(i)Standard enthalpy/heat of displacement ∆Hᶿd
(ii)Standard enthalpy/heat of neutralization ∆Hᶿn
(iii)Standard enthalpy/heat of solution/dissolution ∆Hᶿs
- Standard enthalpy/heat of displacement ∆Hᶿd
The molar standard enthalpy/heat of displacement may be defined as the energy/heat change when one mole of substance is displaced /removed from its solution at standard conditions
Some displacement reactions
(i)Zn(s) + CuSO4(aq) -> Cu(s) + ZnSO4(aq)
Ionically: Zn(s) + Cu2+(aq) -> Cu(s) + Zn2+ (aq)
(ii)Fe(s) + CuSO4(aq) -> Cu(s) + FeSO4(aq)
Ionically: Fe(s) + Cu2+(aq) -> Cu(s) + Fe2+ (aq)
(iii)Pb(s) + CuSO4(aq) -> Cu(s) + PbSO4(s)
This reaction stops after some time as insoluble PbSO4(s) coat/cover unreacted lead.
(iv)Cl2(g) + 2NaBr(aq) -> Br2(aq) + 2NaCl(aq)
Ionically:
Cl2(g)+ 2Br– (aq) -> Br2(aq) + 2Cl– (aq)
To determine the molar standard enthalpy/heat of displacement(∆Hᶿd) of copper
Procedure
Place 20cm3 of 0.2M copper(II)sulphate(VI)solution into a 50cm3 plastic beaker/calorimeter.
Determine and record the temperature of the solution T1.
Put all the Zinc powder provided into the plastic beaker. Stir the mixture using the thermometer.
Determine and record the highest temperature change to the nearest 0.5oC- T2 .
Repeat the experiment to complete table 1 below
Sample results Table 1
| Experiment | I | II |
| Final temperature of solution(T2) | 30.0oC | 31.0oC |
| initial temperature of solution(T1) | 25.0oC | 24.0oC |
| Change in temperature(∆T) | 5.0 | 6.0 |
Questions
1.(a) Calculate:
(i)average ∆T
Average ∆T = change in temperature in experiment I and II
=>5.0 + 6.0 = 5.5oC
2
(ii)the number of moles of solution used
Moles used = molarity x volume of solution = 0.2 x 20 = 0.004 moles
1000 1000
(iii) the enthalpy change ∆H for the reaction
Heat produced ∆H = mass of solution(m) x specific heat capacity (c)x ∆T
=> 20 x 4.2 x 5.5 = 462 Joules = –0.462 kJ
1000 1000
(iv)State two assumptions made in the above calculations.
Density of solution = density of water = 1gcm-3
Specific heat capacity of solution=Specific heat capacity of water =4.2 kJ-1kg-1K
This is because the solution is assumed to be infinite dilute.
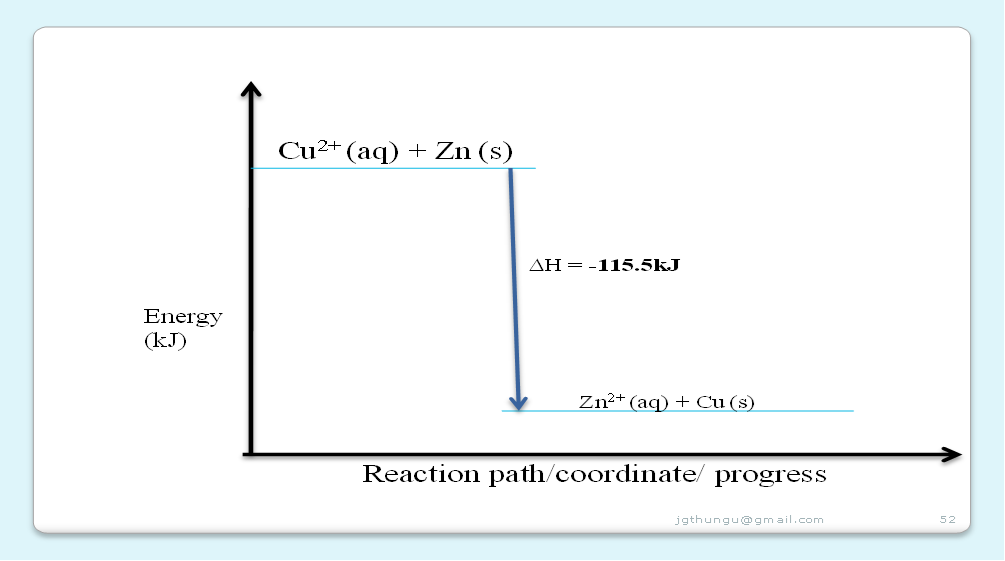
2. Calculate the enthalpy change for one mole of displacement of Cu2+ (aq) ions.
Molar heat of displacement ∆Hd = Heat produced ∆H
Number of moles of fuel
=> 0.462 kJ = –115.5 kJmole-1
0.004
3.Write an ionic equation for the reaction taking place.
Zn(s) + Cu2+(aq) -> Cu(s) + Zn2+(aq)
4.State the observation made during the reaction.
Blue colour of copper(II)sulphate(VI) fades/becomes less blue/colourless.
Brown solid deposits are formed at the bottom of reaction vessel/ beaker.
5.Illustrate the above reaction using an energy level diagram.
8. The enthalpy of displacement ∆Hd of copper(II)sulphate (VI) solution is 12k6kJmole-1.Calculate the molarity of the solution given that 40cm3 of this solution produces 2.204kJ of energy during a displacement reaction with excess iron filings.
Number of moles = Heat produced ∆H
Molar heat of displacement ∆Hd
=> 2.204 kJ = 0.0206moles
126 moles
Molarity of the solution = moles x 1000
Volume of solution used
= 0.0206moles x 1000 = 0.5167 M
40
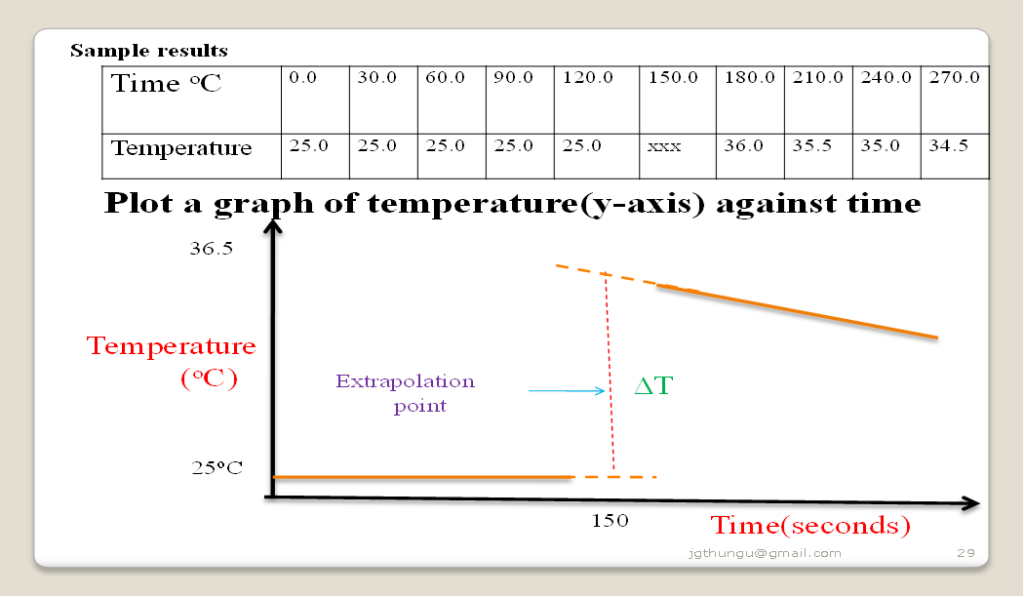
Graphical determination of the molar enthalpy of displacement of copper
Procedure:
Place 20cm3 of 0.2M copper(II)sulphate (VI) solution into a calorimeter/50cm3 of plastic beaker wrapped in cotton wool/tissue paper.
Record its temperature at time T= 0.Stir the solution with the thermometer carefully and continue recording the temperature after every 30 seconds .
Place all the (1.5g) Zinc powder provided after 1 ½ minutes.
Stir the solution with the thermometer carefully and continue recording the temperature after every 30 seconds for five minutes.
Determine the highest temperature change to the nearest 0.5oC.
Questions
1.Show and determine the change in temperature ∆T
From a well constructed graph ∆T= T2 –T1 at 150 second by extrapolation
∆T = 36.5 – 25.0 = 11.5oC
2.Calculate the number of moles of copper(II) sulphate(VI)used given the molar heat of displacement of Cu2+ (aq)ions is 125kJmole-1
Heat produced ∆H = mass of solution(m) x specific heat capacity (c)x ∆T
=> 20 x 4.2 x 11.5 = 966 Joules = –0.966 kJ
1000
Number of moles = Heat produced ∆H Molar heat of displacement ∆Hd
=> 0.966 kJ = –0.007728moles
125 moles –7.728 x 10-3moles
3. What was the concentration of copper(II)sulphate(VI) in moles per litre.
Molarity = moles x 1000
Volume used
=>7.728 x 10-3moles x 1000 = 0.3864M
20
4.The actual concentration of copper
(II) Sulphate (VI) solution was 0.4M. Explain the differences between the two.
Practical value is lower than theoretical
. Heat/energy loss to the surrounding and that absorbed by the reaction vessel decreases ∆T hence lowering the practical number of moles and molarity against the theoretical value
(c)Standard enthalpy/heat of neutralization ∆Hᶿn
The molar standard enthalpy/heat of neutralization ∆Hᶿn is defined as the energy/heat change when one mole of a H+ (H3O+)ions react completely with one mole of OH– ions to form one mole of H2O/water.
Neutralization is thus a reaction of an acid /H+ (H3O+)ions with a base/alkali/ OH– ions to form salt and water only.
Strong acids/bases/alkalis are completely/fully/wholly dissociated to many free ions(H+ /H3O+ and OH– ions).
(ii) for strong acid/base/alkali neutralization, no energy is used to dissociate /ionize since molecule is wholly/fully dissociated/ionized into free H+ H3O+ and OH– ions.
The overall energy evolved is comparatively higher / more than weak acid-base/ alkali neutralizations.
For strong acid-base/alkali neutralization, the enthalpy of neutralization is constant at about 57.3kJmole-1 irrespective of the acid-base used.
This is because ionically:
OH–(aq)+ H+(aq) -> H2O(l)
for all wholly/fully /completely dissociated acid/base/alkali
Weak acids/bases/alkalis are partially dissociated to few free ions(H+ (H3O+ and OH– ions) and exist more as molecules.
Neutralization is an exothermic(-∆H) process.
The energy produced during neutralization depend on the amount of free ions (H+ H3O+ and OH–)ions existing in the acid/base/alkali reactant:
(i)for weak acid-base/alkali neutralization,some of the energy is used to dissociate /ionize the molecule into free H+ H3O+ and OH– ions therefore the overall energy evolved is comparatively lower/lesser/smaller than strong acid / base/ alkali neutralizations.
Practically ∆Hᶿn can be determined as in the examples below:
To determine the molar enthalpy of neutralization ∆Hn of Hydrochloric acid
Procedure
Place 50cm3 of 2M hydrochloric acid into a calorimeter/200cm3 plastic beaker wrapped in cotton wool/tissue paper.
Record its temperature T1.
Using a clean measuring cylinder, measure another 50cm3 of 2M sodium hydroxide.
Rinse the bulb of the thermometer in distilled water.
Determine the temperature of the sodium hydroxide T2.
Average T2 andT1 to get the initial temperature of the mixture T3.
Carefully add all the alkali into the calorimeter/200cm3 plastic beaker wrapped in cotton wool/tissue paper containing the acid.
Stir vigorously the mixture with the thermometer.
Determine the highest temperature change to the nearest 0.5oC T4 as the final temperature of the mixture.
Repeat the experiment to complete table 1.
(ii)enthalpy change ∆H of neutralization.
∆H = (m)mass of solution(acid+base) x (c)specific heat capacity of solution x ∆T(T6) => (50 +50) x 4.2 x 13.5 = 5670Joules = 5.67kJ
(iii) the molar heat of neutralization the acid.
∆Hn = Enthalpy change ∆H => 5.67kJ = 56.7kJ mole-1
Number of moles 0.1moles
(c)Write the ionic equation for the reaction that takes place
OH–(aq)+ H+(aq) -> H2O(l)
(d)The theoretical enthalpy change is 57.4kJ. Explain the difference with the results above.
The theoretical value is higher
Heat/energy loss to the surrounding/environment lowers ∆T/T6 and thus ∆Hn
Heat/energy is absorbed by the reaction vessel/calorimeter/plastic cup lowers ∆T and hence ∆Hn
Sample results
| Experiment | I | II |
| Temperature of acid T1 (oC) | 22.5 | 22.5 |
| Temperature of base T2 (oC) | 22.0 | 23.0 |
| Final temperature of solution T4(oC) | 35.5 | 36.0 |
| Initial temperature of solution T3(oC) | 22.25 | 22.75 |
| Temperature change( T5) | 13.25 | 13.75 |
(a)Calculate T6 the average temperature change T6 = 13.25 +13.75 = 13.5 oC 2
(b)Why should the apparatus be very clean?
Impurities present in the apparatus reacts with acid /base lowering the overall temperature change and hence ∆Hᶿn.
(c)Calculate the:
(i)number of moles of the acid used
number of moles = molarity x volume => 2 x 50 = 0.1moles 1000 1000
(e)Compare the ∆Hn of the experiment above with similar experiment repeated with neutralization of a solution of:
(i) potassium hydroxide with nitric(V) acid
The results would be the same/similar.
Both are neutralization reactions of strong acids and bases/alkalis that are fully /wholly dissociated into many free H+ / H3O+ and OH– ions.
(ii) ammonia with ethanoic acid
The results would be lower/∆Hn would be less.
Both are neutralization reactions of weak acids and bases/alkalis that are partially /partly dissociated into few free H+ / H3O+ and OH– ions. Some energy is used to ionize the molecule.
(f)Draw an energy level diagram to illustrate the energy changes
Theoretical examples
1.The molar enthalpy of neutralization was experimentary shown to be 51.5kJ per mole of 0.5M hydrochloric acid and 0.5M sodium hydroxide. If the volume of sodium hydroxide was 20cm3, what was the volume of hydrochloric acid used if the reaction produced a 5.0oC rise in temperature?
Working:
Moles ofsodium hydroxide = molarity x volume
1000
=> 0.5 M x 20cm3 = 0.01 moles
1000
Enthalpy change∆H = ∆Hn => 51.5 = 0.515kJ
Molessodium hydroxide 0.01 moles
Mass of base + acid = Enthalpy change∆H in Joules
Specific heat capacity x ∆T
=> 0.515kJ x 1000 = 24.5238g
4.2 x 5
Mass/volume of HCl = Total volume – volume of NaOH
=>24.5238 – 20.0 = 4.5238 cm3
Graphically ∆Hn can be determined as in the example below:
Procedure
Place 8 test tubes in a test tube rack .
Put 5cm3 of 2M sodium hydroxide solution into each test tube. Measure 25cm3 of 1M hydrochloric acid into 100cm3 plastic beaker.
Record its initial temperature at volume of base =0.
Put one portion of the base into the beaker containing the acid.
Stir carefully with the thermometer and record the highest temperature change to the nearest 0.5oC.
Repeat the procedure above with other portions of the base to complete table 1 below
| Volume of acid(cm3) | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Volume of alkali(cm3) | 0 | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 | 35.0 | 40.0 |
| Final temperature(oC) | 22.0 | 24.0 | 26.0 | 28.0 | 28.0 | 27.0 | 26.0 | 25.0 | 24.0 | 25.0 | 24.0 |
| Initial temperature(oC) | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
| Change in temperature | 0.0 | 2.0 | 4.0 | 6.0 | 6.0 | 5.0 | 4.0 | 3.0 | 2.0 | 3.0 | 2.0 |
Complete the table to determine the change in temperature.
Plot a graph of volume of sodium hydroxide against temperature change.
From the graph show and determine :
(i)the highest temperature change ∆T
∆T =T2-T1 : highest temperature-T2 (from extrapolating a correctly plotted graph) less lowest temperature at volume of base=0-T1 => 28.7 – 22.0 = 6.7 0 oC
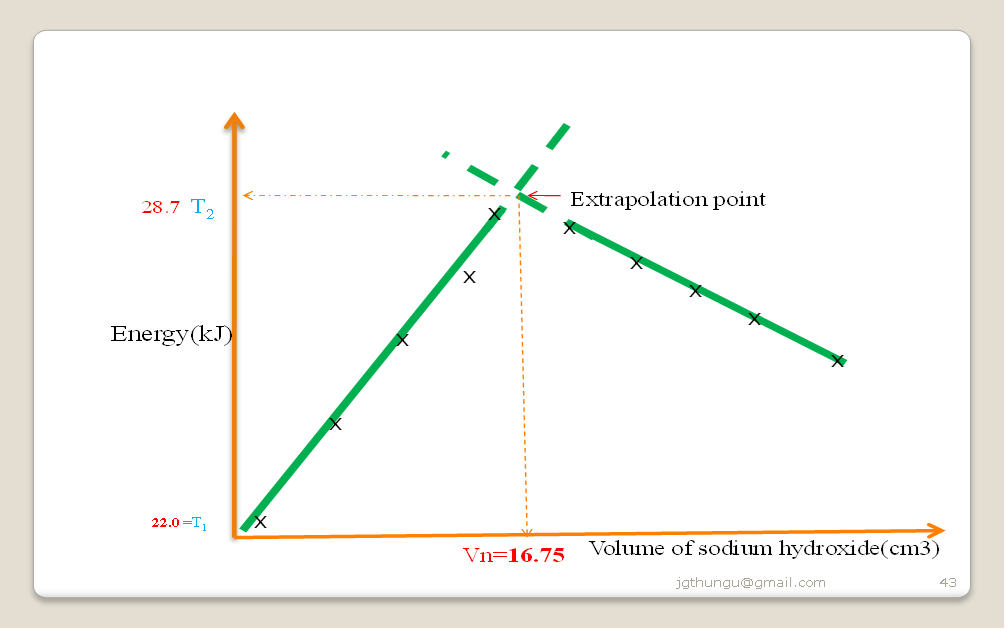
(ii) the volume of sodium hydroxide used for complete neutralization
From correctly plotted graph = 16.75 cm3
(iii) Calculate the number of moles of the alkali used
Moles NaOH = molarity x volume ()Vn=
1000
=> 2 x 16.75 = 0.0335 moles
1000
(iv)Calculate ∆H for the reaction.
∆H = mass of solution mixture x c x ∆T
=> (25.0 + 16.75) x 4.2 x 6.7
= 1174.845 J = 1.174845 kJ
1000
(iii) Calculate the molar enthalpy of the alkali:
∆Hn = Heat change => 1.174845 kJ
number of moles 0.0335 moles
= 35.0699kJ mole-1
(i) Standard enthalpy/heat of solution/dissolution ∆Hᶿs
The standard enthalpy of solution ∆Hᶿs is defined as the energy change when one mole of a substance is dissolved in excess distilled water to form an infinite dilute solution.
An infinite dilute solution is one which is too dilute to be diluted further.
Practically the heat of solution is determined by dissolving a known mass /volume of a solute in known mass/volume of water/solvent and determining the temperature change.
To determine the heat of dissolution of ammonium nitrate(V)
Place 100cm3 of distilled water into a plastic cup/beaker/calorimeter
Put all the 5.0g of ammonium nitrate(v)/potassium nitrate(V)/ ammonium chloride into the water.
Stir the mixture using the thermometer and record the temperature change after every ½ minute to complete table1.
Continue stirring throughout the experiment.
(a)From the graph show and determine:
(i)the highest temperature change ∆T
∆T =T2-T1 : highest temperature-T2 (from extrapolating a correctly plotted graph) less lowest temperature at volume of base=0-T1
=> 18.7 – 22.0 = 3.3 oC ( not -3.3 oC)
(b) Calculate the total energy change ∆H during the reaction
∆H= mass of water x c x ∆T
=>∆H=100 x4.2 x 3.3 oC = + 1386 J = + 1.386 kJ
1000
(c) Calculate the number of moles of ammonium nitrate (v) used
Moles = mass => 5.0 = 0.0625 moles
molar mass 80
(d)What is the molar heat of dissolution of ammonium nitrate(V)
∆H = Heat change = + 1.386 kJ = + 22.176 kJmole-1
Number of mole 0.0625 moles
(e)What would happen if the distilled water is heated before experiment was performed .
The ammonium nitrate(V) would take less time to dissolve.
Increase in temperature reduces lattice energy causing endothermic dissollution to be faster.
(e)Illustrate the above process on an energy level diagram
Practical: Chemical Rate of reaction
The rate of a chemical reaction can be defined as the time taken for a known amount of reactants to form known amount of products.
Some reactions are too slow to be determined e.g weathering others are instantaneous
The SI unit of time is seconds. Minutes and hours are also common .
Time is determined using a stop watch/clock
Candidates using stop watch/clock should learn to:
(i)Press start button concurrently with starting off determination of a reaction using one hand each.
(ii)Press stop button when the reaction is over.
(iii)Record all times in seconds unless specified.
(iv)Press reset button to begin another timing
(v)Ignore time beyond seconds for stop clock/watch beyond this accuracy
(vi)Avoid accidental pressing of any button before recording
It can be very frustrating repeating a whole procedure
The following factors theoretically and practically alter/influence/affect/determine the rate of a chemical reaction:
(a)Concentration
(b)Temperature
(a)Concentration
An increase in concentration increases the rate the rate of reaction by reducing the time taken to completion.
Theoretically, increase in concentration is a decrease in distance between reacting particles which increases their collision frequency.
Practically decreasing concentration is diluting/adding water
To demonstrate the effect of concentration on reaction rate
You are provided with
(i) sodium thiosulphate containing 40gdm –3 solution labeled A
(ii) 2M hydrochloric acid labeled solution B
You are required to determine the rate of reaction between solution A and B
Procedure
Measure 40cm3 of solution A into 100 cm3 glass beaker. Place it on top of a pen-mark “X”. Measure another 40cm3 of solution B. Simultaneously put solution B into solution A and start off a stop watch/clock. Determine the time taken for the pen-mark “X” to be invisible/obscurred from above. Repeat the procedure by measuring 35cm3 of solution B and adding 5cm3 of water. Complete the table 1 below by using other values os solution B and water
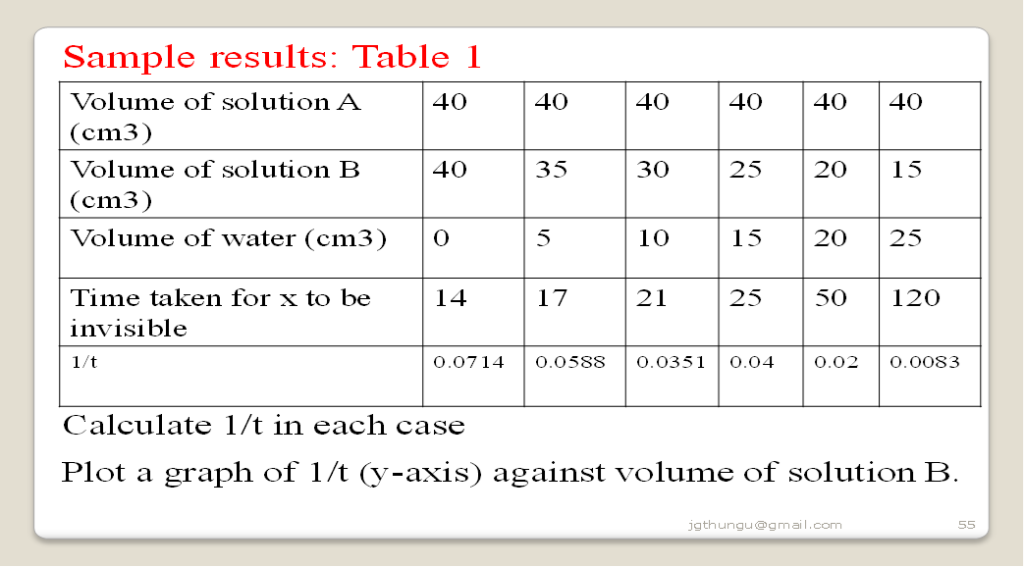
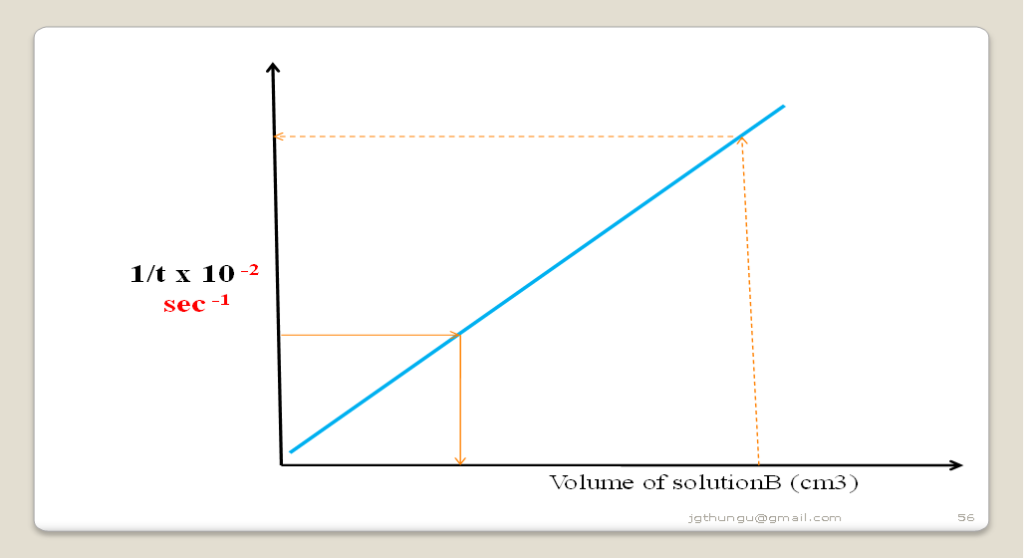
Sample questions
(i)Explain the shape of the graph
(Straight line graph from the origin)
Decrease in concentration decreases the rate of reaction. The higher the concentration of solution B the less time taken for mark x to be obscurred/invisible due to increased collision frequency between the reacting particles.
(ii)From the graph determine the time taken for the mark to be invisible at 37cm3
At 37cm3 then 1/t => 1/ 37 = 0.027
From a well plotted graph:
1/t = 0.027 => 16.2602 seconds
(ii)From the graph determine the volume of solution B at 100 seconds
100 seconds => 1/t = 1 / 1000 = 0.01
From a well plotted graph:
At 1/t = 0.01 => the volume of B = 17.0cm3
(iii) State another factor that would alter the rate of the above reaction.
Temperature
(iii) State another factor that would not alter the rate of the above reaction.
Surface area
Pressure
Catalyst
(b) Temperature
An increase in temperature increases the rate of reaction.
An increase of 10 oC/10K practically doubles the rate of a chemical reaction/reduces time of completion by 1/2.
An increase in temperature increase the kinetic energy of reacting particles increasing their collision frequency
Practically ,increase in temperature involves heating the reactants
The results and presentation should be as in the effect of concentration.
Increased temperature reverses the table I time results
i.e less time as temperature increases.
Chem Practicals: Qualitative Analysis
Process of identifying unknown compounds
Compounds may be:
(i)Inorganic
(ii)organic
Inorganic analysis:
This involve mainly identification of ionic compounds containing cations and anions.
Cations present in an ionic compounds are identified by adding a precipitating reagent that forms a precipitate unique to the cation/s in the compound.
The main precipitating reagents used are:
2M NaOH and/or 2M NH3(aq)
When using 2M sodium hydroxide:
(i)No white precipitate is formed if K + and Na + ions are present
(ii) No white precipitate is formed if NH4+ ions are present but a clourless gas with pungent smell of urine is produced which may not be recognized in a school laboratory examination setting.
(iii)White precipitate that dissolves / soluble in excess if Zn2+ Pb2+ Al3+ ions are present.
(iv)White precipitate that do not dissolves/insoluble in excess if Ba2+ Mg2+ Ca2+ ions are present.
(v)Blue precipitate that do not dissolves /insoluble in excess if Cu2+ ions are present.
(vi)Green precipitate that do not dissolves/insoluble in excess if Fe2+ ions are present.
(vii)Brown precipitate that do not dissolves/insoluble in excess if Fe3+ ions are present.
When using 2M aqueous ammonia
(i)No white precipitate is formed if K + ,NH4+ Na + ions are present
(ii)White precipitate that dissolves / soluble in excess if Zn2+ ions are present.
(iii)White precipitate that do not dissolves/insoluble in excess if Ba2+ Mg2+ Ca2+ Pb2+ Al3+ ions are present.
(iv)Blue precipitate that dissolves /soluble in excess to form a deep/royal blue solution in excess if Cu2+ ions are present.
(v)Green precipitate that do not dissolves/insoluble in excess if Fe2+ ions are present.
(vi)Brown precipitate that do not dissolves/insoluble in excess if Fe3+ ions are present.
Anions present in an ionic compounds are identified by adding a specific precipitating reagent that forms a precipitate unique to the specific anion/s in the compound.
(i)Lead(II)nitrate(V) solution
Lead forms insoluble PbSO4 ,PbSO3 ,PbCO3, PbS, PbI2,PbCl2
PbS is a black precipitate,
PbI2 is a yellow precipitate.
All the others are white precipitates.
(a)If a Lead(II)nitrate(V) solution is added to a substance/ solution/ compound :
(i)A yellow ppt shows presence of I– ions
(ii)A black ppt shows presence of S2- ions
(iii) A white ppt shows presence of SO42- ,SO32- ,CO32- Cl–
(b)If the white precipitate is added dilute nitric(V) acid:
(i)It dissolves to show presence of SO32- ,CO32-
(ii)It persist/remains to show presence of SO42-, Cl–
(c)If the white precipitate in b(i) is added acidified potassium manganate(VII)/ dichromate(VI)
(i) acidified potassium manganate(VII) is decolorized /orange colour of acidified potassium dichromate(VI) turns to green to show presence of SO32-
(ii) acidified potassium manganate(VII) is not decolorized /orange colour of acidified potassium dichromate(VI) does not turn to green/remains orange to show absence of SO32- /presence of CO32-
(c)If the white precipitate in b(ii) is boiled:
(i)It dissolves to show presence of Cl–
(ii)It persist/remains to show presence of SO42-
(ii)Barium(II)nitrate(V)/Barium chloride solution
Barium(II)nitrate(V)/Barium chloride solution precipitates BaSO4 ,BaSO3 , BaCO3, from SO42- ,SO32- ,CO32- ions.
Inorganic qualitative analysis require continous practice discussion
Sample presentation of results
You are provided with solid Y(aluminium (III)sulphate(VI)hexahydrate).Carry out the following tests and record your observations and inferences in the space provided.
1(a) Appearance
Observations inference (1mark)
White crystalline solid Coloured ions Cu2+ , Fe2+ ,Fe3+ absent
(b)Place about a half spatula full of the solid into a clean dry boiling tube. Heat gently then strongly.
Observations inference (1mark)
Colourless droplets formed on the cooler Hydrated compound/compound
part of the test tube containing water of crystallization
Solid remains a white residue
(c)Place all the remaining portion of the solid in a test tube .Add about 10cm3 of distilled water. Shake thoroughly. Divide the mixture into five portions.
Observation Inference (1mark)
Solid dissolves to form Polar soluble compound
a colourless solution Cu2+ , Fe2+ ,Fe3+ absent
(i)To the first portion, add three drops of sodium hydroxide then add excess of the alkali.
Observation Inference (1mark)
White ppt, soluble in excess Zn2+ , Pb2+ , Al3+
(ii)To the second portion, add three drops of aqueous ammonia then add excess of the alkali.
Observation Inference (1mark)
White ppt, insoluble in excess Pb2+ , Al3+
(iii)To the third portion, add three drops of sodium sulphate(VI)solution.
Observation Inference (1mark)
No white ppt Al3+
(iv)I.To the fourth portion, add three drops of Lead(II)nitrate(IV)solution. Preserve
Observation Inference (1mark)
White ppt CO32-, SO42-, SO32-, Cl–,
II.To the portion in (iv) I above , add five drops of dilute hydrochloric acid.
Observation Inference (1mark)
White ppt persist/remains SO42-, Cl–,
III.To the portion in (iv) II above, heat to boil.
Observation Inference (1mark)
White ppt persist/remains SO42-,
Organic analysis:
This involve mainly identification of the functional group:
(i) – C = C- / = C = C= / C – C
- R OH
(iii) R COOH / H+
These functional groups can be identified by:
(i)burning-a substance which “catches fire” must reduce in amount.
Candidates should not confuse burning with flame coloration/test
(ii)Decolorization of bromine water/chlorine water/acidified KMnO 4 / to show presence of
- C = C – / – C = C – and R – OH
(iii)Turning orange acidified K 2 Cr 2 O 7to green to show presence as in above.
(iii)pH 1/2/3 for strongly acidic solutions. pH 4/5/6 for weakly acidic solutions
(iv)Turning blue litmus paper red. red litmus paper remaining red show presence of H+ ions
d)Flame test
The colour change on a clear colourless Bunsen flame is useful in identifying some cations / metals.
A very clean metallic spatula is recommended since dirt obscures /changes the correct coloration distinct flame coloration of some compounds
| Barium/barium salts | orange |
| Sodium/ sodium salts | yellow |
| Potassium/potassium salts | Purple/lilac |
| Lithium/Lithium salts | Deep red/crimson |
| Calcium/ calcium salts | red |
| Copper/copper salts | Blue/ green |
(e)Physical chemistry
Chemistry is a science subject that incorporate many scientific techniques.
Examining body/council, require tabulated results/data from the candidate.
This tabulated results is usually then put in a graph.
The general philosophy of methods of presentation of chemistry practical data is therefore availability of evidence showing:
(i)Practical done(complete table)
(ii)Accuracy of apparatus used(decimal point)
(iii)Accuracy/care in doing experiment to get collect trend(against teachers results)
(iv)Graphical work(use of mathematical science)
(v)Calculations (Scientific mathematical integration)
