(c)Properties of Sulphur(IV)oxide(Questions)
1. Write the equations for the reaction for the formation of sulphur (IV)oxide using:
(i)Method 1
Cu(s) + 2H2SO4(l) -> CuSO4(aq) + SO2(g) + 2H2O(l)
Zn(s) + 2H2SO4(l) -> ZnSO4(aq) + SO2(g) + 2H2O(l)
Mg(s) + 2H2SO4(l) -> MgSO4(aq) + SO2(g) + 2H2O(l)
Fe(s) + 2H2SO4(l) -> FeSO4(aq) + SO2(g) + 2H2O(l)
Calcium ,Lead and Barium will form insoluble sulphate(VI)salts that will cover unreacted metals stopping further reaction thus producing very small amount/quantity of sulphur (IV)oxide gas.
(ii)Method 2
Na2SO3(aq) + HCl(aq) -> NaCl(aq ) + SO2(g) + 2H2O(l)
K2SO3(aq) + HCl(aq) -> KCl(aq ) + SO2(g) + 2H2O(l)
BaSO3(s) + 2HCl(aq) -> BaCl2(aq ) + SO2(g) + H2O(l)
CaSO3(s) + 2HCl(aq) -> CaCl2(aq ) + SO2(g) + H2O(l)
PbSO3(s) + 2HCl(aq) -> PbCl2(s ) + SO2(g) + H2O(l)
Lead(II)chloride is soluble on heating thus reactants should be heated to prevent it coating/covering unreacted PbSO3(s)
2.State the physical properties unique to sulphur (IV)oxide gas.
Sulphur (IV)oxide gas is a colourless gas with a pungent irritating and choking smell which liquidifies easily. It is about two times denser than air.
3. The diagram below show the solubility of sulphur (IV)oxide gas. Explain.
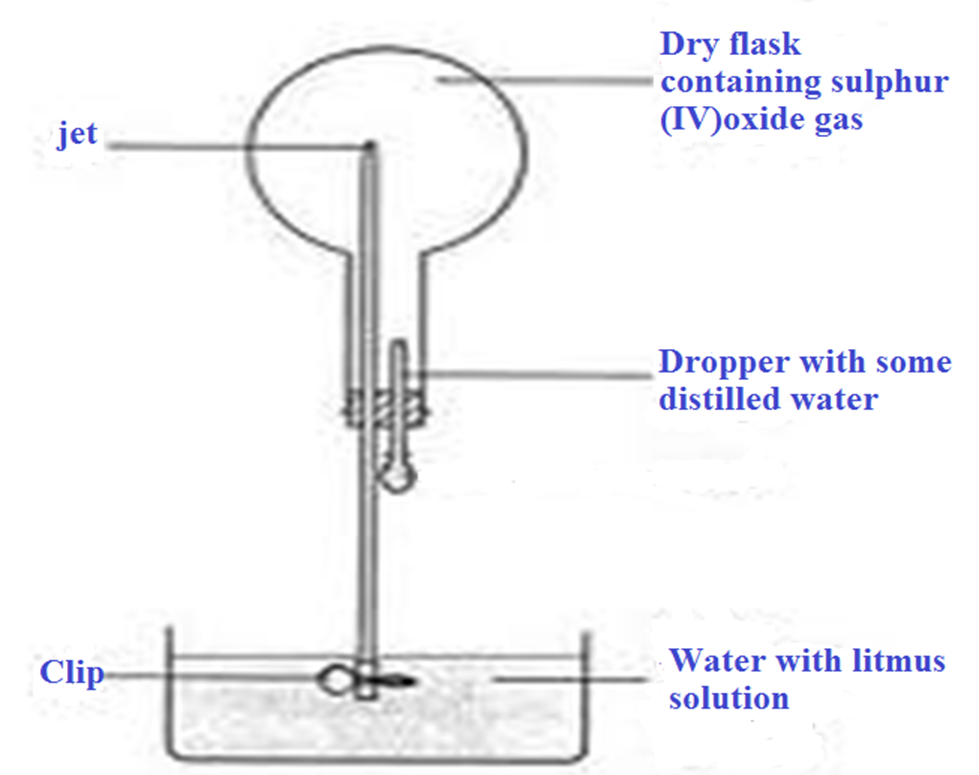
Sulphur(IV) oxide is very soluble in water.
One drop of water dissolves all the Sulphur (IV) oxide in the flask leaving a vacuum.
If the clip is removed, atmospheric pressure forces the water up through the narrow tube to form a fountain to occupy the vacuum.
An acidic solution of sulphuric (IV)acid is formed which turns litmus solution red.
Chemical equation
SO2(g) + H2O(l) -> H2 SO3 (aq) ( sulphuric(IV)acid turn litmus red)
4.Dry litmus papers and wet/damp/moist litmus papers were put in a gas jar containing sulphur(IV) oxide gas. State and explain the observations made.
Observations
(i)Dry Blue litmus paper remains blue.
Dry red litmus paper remains red.
(ii) Wet/damp/moist blue litmus paper turns red.
Moist/damp/wet red litmus paper remains red.
Both litmus papers are then bleached /decolorized.
Explanation
Dry sulphur(IV) oxide gas is a molecular compound that does not dissociate/ionize to release H+(aq)ions and thus has no effect on dry blue/red litmus papers.
Wet/damp/moist litmus papers contain water that dissolves /react with dry sulphur(IV) oxide gas to form a solution of weak sulphuric(IV)acid (H2 SO3 (aq)).
Weak sulphuric(IV)acid(H2 SO3 (aq)) dissociates /ionizes into free H+(aq)ions:
H2 SO3 (aq) -> 2H+(aq) + SO32- (aq)
The free H+(aq)ions are responsible for turning blue litmus paper turns red showing the gas is acidic.
The SO32- (aq) ions in wet/damp/moist sulphur(IV) oxide gas is responsible for many reactions of the gas.
It is easily/readily oxidized to sulphate(VI) SO42- (aq) ions making sulphur(IV) oxide gas act as a reducing agent as in the following examples:
(a)Bleaching agent
Wet/damp/moist coloured flowers/litmus papers are bleached/decolorized when put in sulphur(IV) oxide gas.
This is because sulphur(IV) oxide removes atomic oxygen from the coloured dye/ material to form sulphuric(VI)acid.
Chemical equations
(i)Formation of sulphuric(IV)acid
SO2(g) + H2O(l) -> H2 SO3 (aq)
(ii)Decolorization/bleaching of the dye/removal of atomic oxygen.
Method I. H2 SO3 (aq) + (dye + O) -> H2 SO4 (aq) + dye
(coloured) (colourless)
Method II. H2 SO3 (aq) + (dye) -> H2 SO4 (aq) + (dye – O)
(coloured) (colourless)
Sulphur(IV) oxide gas therefore bleaches by reduction /removing oxygen from a dye unlike chlorine that bleaches by oxidation /adding oxygen.
The bleaching by removing oxygen from Sulphur(IV) oxide gas is temporary.
This is because the bleached dye regains the atomic oxygen from the atmosphere/air in presence of sunlight as catalyst thus regaining/restoring its original colour. e.g.
Old newspapers turn brown on exposure to air on regaining the atomic oxygen.
The bleaching through adding oxygen by chlorine gas is permanent.
(b)Turns Orange acidified potassium dichromate(VI) to green
Experiment:
(i)Pass a stream of Sulphur(IV) oxide gas in a test tube containingacidified potassium dichromate(VI) solution. or;
(ii)Dip a filter paper soaked in acidified potassium dichromate(VI) into a gas jar containing Sulphur(IV) oxide gas.
Observation:
Orange acidified potassium dichromate(VI) turns to green.
Explanation:
Sulphur(IV) oxide gas reduces acidified potassium dichromate(VI) from orange Cr2O72- ions to green Cr3+ ions without leaving a residue itself oxidized from SO32- ions in sulphuric(IV) acid to SO42- ions in sulphuric(VI) acid.
Chemical/ionic equation:
(i)Reaction of Sulphur(IV) oxide gas with water
SO2(g) + H2O(l) -> H2 SO3 (aq)
(ii)Dissociation /ionization of Sulphuric(IV)acid.
H2 SO3 (aq) -> 2H+(aq) + SO32- (aq)
(iii)Oxidation of SO32- (aq)and reduction of Cr2O72-(aq)
3SO32-(aq) + Cr2O72-(aq) +8H+(aq) -> 3SO42-(aq) + 2Cr3+(aq) + 4H2O(l)
This is a confirmatory test for the presence of Sulphur(IV) oxide gas.
Hydrogen sulphide also reduces acidified potassium dichromate(VI) from orange Cr2O72- ions to green Cr3+ ions leaving a yellow residue.
(c)Decolorizes acidified potassium manganate(VII)
Experiment:
(i)Pass a stream of Sulphur(IV) oxide gas in a test tube containingacidified potassium manganate(VII) solution. or;
(ii)Dip a filter paper soaked in acidified potassium manganate(VII) into a gas jar containing Sulphur(IV) oxide gas.
Observation:
Purple acidified potassium manganate(VII) turns to colourless/ acidified potassium manganate(VII) is decolorized.
Explanation:
Sulphur(IV) oxide gas reduces acidified potassium manganate(VII) from purple MnO4– ions to green Mn2+ ions without leaving a residue itself oxidized from SO32- ions in sulphuric(IV) acid to SO42- ions in sulphuric(VI) acid.
Chemical/ionic equation:
(i)Reaction of Sulphur(IV) oxide gas with water
SO2(g) + H2O(l) -> H2 SO3 (aq)
(ii)Dissociation /ionization of Sulphuric(IV)acid.
H2 SO3 (aq) -> 2H+(aq) + SO32- (aq)
(iii)Oxidation of SO32- (aq)and reduction of MnO4– (aq)
5SO32-(aq) + 2MnO4– (aq) +6H+(aq) -> 5SO42-(aq) + 2Mn2+(aq) + 3H2O(l)
(purple) (colourless)
This is another test for the presence of Sulphur(IV) oxide gas.
Hydrogen sulphide also decolorizes acidified potassium manganate(VII) from purple MnO4– ions to colourless Mn2+ ions leaving a yellow residue.
(d)Decolorizes bromine water
Experiment:
(i)Pass a stream of Sulphur(IV) oxide gas in a test tube containingbromine water . or;
(ii)Put three drops of bromine water into a gas jar containing Sulphur(IV) oxide gas. Swirl.
Observation:
Yellow bromine water turns to colourless/ bromine water is decolorized.
Explanation:
Sulphur(IV) oxide gas reduces yellow bromine water to colourless hydrobromic acid (HBr) without leaving a residue itself oxidized from SO32- ions in sulphuric (IV) acid to SO42- ions in sulphuric(VI) acid.
Chemical/ionic equation:
(i)Reaction of Sulphur(IV) oxide gas with water
SO2(g) + H2O(l) -> H2 SO3 (aq)
(ii)Dissociation /ionization of Sulphuric(IV)acid.
H2 SO3 (aq) -> 2H+(aq) + SO32- (aq)
(iii)Oxidation of SO32- (aq)and reduction of MnO4– (aq)
SO32-(aq) + Br2 (aq) + H2O(l) -> SO42-(aq) + 2HBr(aq)
(yellow) (colourless)
This can also be used as another test for the presence of Sulphur(IV) oxide gas.
Hydrogen sulphide also decolorizes yellow bromine water to colourless leaving a yellow residue.
(e)Reduces Iron(III) Fe3+ salts to Iron(II) salts Fe2+
Experiment:
(i)Pass a stream of Sulphur(IV) oxide gas in a test tube containingabout 3 cm3 of Iron (III)chloride solution. or;
(ii)Place about 3cm3 of Iron (III)chloride solution into a gas jar containing Sulphur(IV) oxide gas.Swirl.
Observation:
Yellow/brown Iron (III)chloride solution turns to green
Explanation:
Sulphur(IV) oxide gas reduces Iron (III)chloride solution from yellow/brown Fe3+ ions to green Fe2+ ions without leaving a residue itself oxidized from SO32- ions in sulphuric(IV) acid to SO42- ions in sulphuric(VI) acid.
Chemical/ionic equation:
(i)Reaction of Sulphur(IV) oxide gas with water
SO2(g) + H2O(l) -> H2 SO3 (aq)
(ii)Dissociation /ionization of Sulphuric(IV)acid.
H2 SO3 (aq) -> 2H+(aq) + SO32- (aq)
(iii)Oxidation of SO32- (aq)and reduction of Fe3+ (aq)
SO32-(aq) + 2Fe3+ (aq) +3H2O(l) -> SO42-(aq) + 2Fe2+(aq) + 2H+(aq)
(yellow) (green)
(f)Reduces Nitric(V)acid to Nitrogen(IV)oxide gas
Experiment:
(i)Pass a stream of Sulphur(IV) oxide gas in a test tube containingabout 3 cm3 of concentrated nitric(V)acid. or;
(ii)Place about 3cm3 of concentrated nitric(V)acid into a gas jar containing Sulphur(IV) oxide gas. Swirl.
Observation:
Brown fumes of a gas evolved/produced.
Explanation:
Sulphur(IV) oxide gas reduces concentrated nitric(V)acid to brown nitrogen(IV)oxide gas itself oxidized from SO32- ions in sulphuric(IV) acid to SO42- ions in sulphuric(VI) acid.
Chemical/ionic equation:
SO2(g) + 2HNO3 (l) -> H2 SO4 (l) + NO2 (g)
(brown fumes/gas)
(g)Reduces Hydrogen peroxide to water
Experiment:
(i)Pass a stream of Sulphur(IV) oxide gas in a test tube containingabout 3 cm3 of 20 volume hydrogen peroxide. Add four drops of Barium nitrate(V)or Barium chloride followed by five drops of 2M hydrochloric acid/ 2M nitric(V) acid.
Observation:
A white precipitate is formed that persist /remains on adding 2M hydrochloric acid/ 2M nitric(V) acid.
Explanation:
Sulphur(IV) oxide gas reduces 20 volume hydrogen peroxide and itself oxidized from SO32- ions in sulphuric(IV) acid to SO42- ions in sulphuric(VI) acid.
When Ba2+ ions in Barium Nitrate(V) or Barium chloride solution is added, a white precipitate of insoluble Barium salts is formed showing the presence of of either SO32- ,SO42- ,CO32- ions. i.e.
Chemical/ionic equation:
SO32-(aq) + Ba2+ (aq) -> BaSO3(s)
white precipitate
SO42-(aq) + Ba2+ (aq) -> BaSO4(s)
white precipitate
CO32-(aq) + Ba2+ (aq) -> BaCO3(s)
white precipitate
If nitric(V)/hydrochloric acid is added to the three suspected insoluble white precipitates above, the white precipitate:
(i) persist/remains if SO42-(aq)ions (BaSO4(s)) is present.
(ii)dissolves if SO32-(aq)ions (BaSO3(s)) and CO32-(aq)ions (BaCO3(s))is present. This is because:
I. BaSO3(s) reacts with Nitric(V)/hydrochloric acid to produce acidic SO2 gas that turns Orange moist filter paper dipped in acidified Potassium dichromate to green.
Chemical equation
BaSO3(s) +2H+(aq) -> Ba2+ (aq) + SO2(g) + H2O(l)
I. BaCO3(s) reacts with Nitric(V)/hydrochloric acid to produce acidic CO2 gas that forms a white precipitate when bubbled in lime water.
Chemical equation
BaCO3(s) +2H+(aq) -> Ba2+ (aq) + CO2(g) + H2O(l)
5.Sulphur(IV)oxide also act as an oxidizing agent as in the following examples.
(a)Reduction by burning Magnesium
Experiment
Lower a burning Magnesium ribbon into agas jar containing Sulphur(IV)oxide gas
Observation
Magnesium ribbon continues to burn with difficulty.
White ash and yellow powder/speck
Explanation
Sulphur(IV)oxide does not support burning/combustion. Magnesium burns to produce enough heat energy to decompose Sulphur(IV)oxide to sulphur and oxygen.
The metal continues to burn on Oxygen forming white Magnesium oxide solid/ash.
Yellow specks of sulphur residue form on the sides of reaction flask/gas jar.
During the reaction, Sulphur(IV)oxide is reduced(oxidizing agent)while the metal is oxidized (reducing agent)
Chemical equation
SO2(g) + 2Mg(s) -> 2MgO(s) + S(s)
(white ash/solid) (yellow speck/powder)
(b)Reduction by Hydrogen sulphide gas
Experiment
Put two drops of water into a gas jar containing dry Sulphur(IV)oxide gas
Bubble hydrogen sulphide gas into the gas jar containing Sulphur(IV)oxide gas.
Or
Put two drops of water into a gas jar containing dry Sulphur(IV)oxide gas
Invert a gas jar full of hydrogen sulphide gas over the gas jar containing Sulphur(IV)oxide gas. Swirl
Observation
Yellow powder/speck
Explanation
Sulphur(IV)oxide oxidizes hydrogen sulphide to yellow specks of sulphur residue and itself reduced to also sulphur that form on the sides of reaction flask/gas jar.
A little moisture/water act as catalyst /speeds up the reaction.
Chemical equation
SO2(g) + 2H2S(g) -> 2H2O(l) + 3S(s)
(yellow speck/powder)
6.Sulphur(IV)oxide has many industrial uses. State three.
(i)In the contact process for the manufacture of Sulphuric(VI)acid
(ii)As a bleaching agent of pulp and paper.
(iii)As a fungicide to kill microbes’
(iv)As a preservative of jam, juices to prevent fermentation
(ii) Sulphur(VI)oxide(SO3)
(a) Occurrence
Sulphur (VI)oxide is does not occur free in nature/atmosphere
(b) Preparation
In a Chemistry school laboratory Sulphur (VI)oxide may prepared from:
Method 1;Catalytic oxidation of sulphur(IV)oxide gas.
Sulphur(IV)oxide gas and oxygen mixture are first dried by being passed through Concentrated Sulphuric(VI)acid .
The dry mixture is then passed through platinised asbestos to catalyse/speed up the combination to form Sulphur (VI)oxide gas.
Sulphur (VI)oxide gas readily solidify as silky white needles if passed through a freezing mixture /ice cold water.
The solid fumes out on heating to a highly acidic poisonous gas.
Chemical equation
2SO2(g) + O2(g) –platinised asbestos–> 2SO3 (g)
Method 2; Heating Iron(II)sulphate(VI) heptahydrate
When green hydrated Iron(II)sulphate(VI) heptahydrate crystals are heated in a boiling tube ,it loses the water of crystallization and colour changes from green to white.
Chemical equation
FeSO4.7H2O(s) -> FeSO4(s) + 7H2O(l)
(green solid) (white solid)
On further heating ,the white anhydrous Iron(II)sulphate(VI) solid decomposes to a mixture of Sulphur (VI)oxide and Sulphur (IV)oxide gas.
Sulphur (VI) oxide readily / easily solidify as white silky needles when the mixture is passed through a freezing mixture/ice cold water.
Iron(III)oxide is left as a brown residue/solid.
Chemical equation
2FeSO4 (s) -> Fe2O3(s) + SO2 (g) + SO3(g)
(green solid) (brown solid)
Caution
On exposure to air Sulphur (VI)oxide gas produces highly corrosive poisonous fumes of concentrated sulphuric(VI)acid and thus its preparation in a school laboratory is very risky.
(c) Uses of sulphur(VI)oxide
One of the main uses of sulphur(VI)oxide gas is as an intermediate product in the contact process for industrial/manufacture/large scale/production of sulphuric(VI)acid.
(iii) Sulphuric(VI)acid(H2SO4)
(a) Occurrence
Sulphuric (VI)acid(H2SO4) is one of the three mineral acids.There are three mineral acids;
Nitric(V)acid
Sulphuric(VI)acid
Hydrochloric acid.
Mineral acids do not occur naturally but are prepared in a school laboratory and manufactured at industrial level.
