CHEMISTRY Form 2: Practice 1: Time 2 hours 80 marks
1. Study the information in the table below and answer the questions that follow (The letters do not represent the actual symbols of the elements)
| Ionization Energy_kJ/Mole | |||
| Element | Electronic configuration | 1st ionization energy | 2nd ionization energy |
| A | 2.2 | 900 | 1800 |
| B | 2.8.2 | 736 | 1450 |
| C | 2.8.8.2 | 590 | 1150 |
(i) What chemical family do the elements A, B and C belong? (1mk)
(ii)Write the formula and electronic structure of an ion of B(2mks)
Formula
Electronic structure
(iii)What type of bonding exist in(2mks)
- atoms of C
- chloride of B
(iv)What is ionization energy(1mk)
(v)Explain the following:
1.The 1st ionization energy is lower that the second ionization energy.(2mk)
2.The 1st ionization energy of B is lower that of C.(2mk)
(vi)Write a chemical equation for the reaction of element B with:
1.Air
2.Chlorine gas
3.Steam(water vapour)
2.Study the information in the table below and answer the questions that follow ( the letters do not represent the actual symbol of the substances)
| Substance | Melting Point (0C) | Boiling Point (0C) | Solubility in water | Density at room temperature g/cm3 |
| H J K L | -117 -78 -23 -219 | 78.5 -33 77 -183 | Very soluble Very soluble Insoluble Slightly soluble | 0.8 0.77 x 10-3 1.6 1.33 x 10-3 |
(i) Which substance would dissolve in water and could be separated from the solution by fractional distillation? Give a reason (2mk)
(ii) Which substances is a liquid at room temperature and when mixed with water two layers would be formed? Explain (2mk)
(iii) Which letter represents a substance that is gas at room temperature and which can be collected:
I. Over water? Explain (2mk)
II. By downward displacement of air? (Density of air is 1.29 x 10-3g/cm3 at room temperature).
Explain (2mk)
3. The grid below represents part of the periodic table. The letters do not represent the actual symbols.
| A | ||||||||
| B | X | G | P | Z | E | V | ||
| J | I | L | R | T | ||||
| D | G | M |
a) Select the most reactive
(i)non-metal. (1mk)
(ii)metal. (1mk)
b) Write the formula of the compound consisting of (10mk)
1.D and Z only.
2 . X and Z only
3. Oxide of B
4. Carbonate of J
5. sulphate of D
6. Nitrate of B
7. Chloride of X
8. Sodium compound of E
9. Aluminium compound of Z
10. Hydrogen compound of G
c) Select an element that can form an ion of charge (10mk)
(i) +1
(ii) -1
(iii) +2
(iv) +3
(v) -3
d) Which element has the least ionization energy? Explain (2mks)
f) To which chemical family do the following elements belong? (3mk)
J
E
B
(g)When a piece of element G is placed in cold water, it sinks to the bottom and effervescence of a colourless gas that burns explosively is produced. Use a simple diagram to illustrate how this gas can be collected during this experiment. (3mks)
h) An element K has relative atomic mass of 40.2.It has two isotopes of masses 39 and 42. Calculate the relative abundance of each isotope. (3mks)
4. Balance the following chemical equation (6mk)
1.Ca (OH)2(aq) + Cl2(g) → CaCl2(aq) + CaOCl2(aq) + H2O(l)
2.NaOH + Cl2(g) → NaClO3 (aq) + NaCl(aq) + 3H2O(l)
3.NaCl(s) + H2SO4(l) -> NaHSO4(aq) + HCl(g)
4.CaO(s) + H2O(l) -> Ca(OH)2(aq) + H2O(l)
5.Fe(s) + HCl(aq) -> FeCl3(aq) + H2 (g)
6.Zn(s) + HCl(aq) -> ZnCl2(aq + H2 (g)
5.The diagram below shows a set up of apparatus for the school laboratory collection of dry chlorine gas.
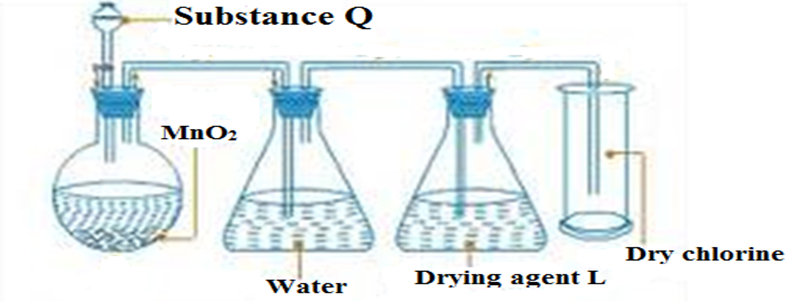
A) Name (2mk)
(i) Substance Q
(ii) Suitable drying agent L
b) State a missing condition for the reaction to take place faster. (1mk)
c) Moist red and blue litmus papers were dipped into the chlorine gas from the above set up .State and explain the observations made. (2mk)
d) Write the equation for the reaction taking place in the conical flask (1mk)
e) Name two other substances that can be used in place of MnO2 (2mk)
(f)State three uses of chlorine (3mk)
6. Study the set up below.
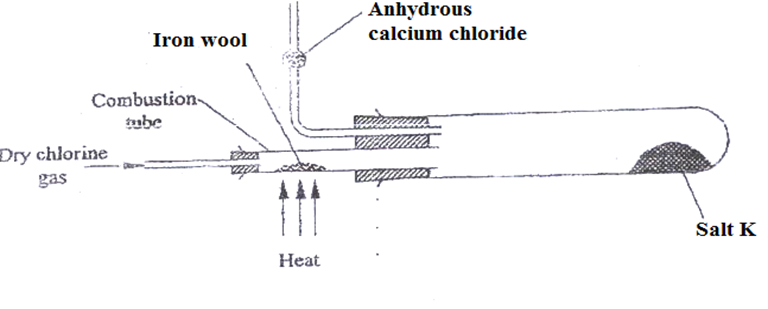
a) Name salt K (1mk)
(b)Write the equation for the reaction for the formation of salt K (1mk)
(c)What property of salt A is exhibited as shown in the experiment.(1mk)
(d)What is the purpose of anhydrous calcium chloride? Explain (2mk)
(e)Name another metal that can be used to produce similar results (1mk)
7. In an experiment, dry hydrogen chloride gas was passed through heated zinc turnings as in the set up below. The gas produced was the passed through copper (II) oxide
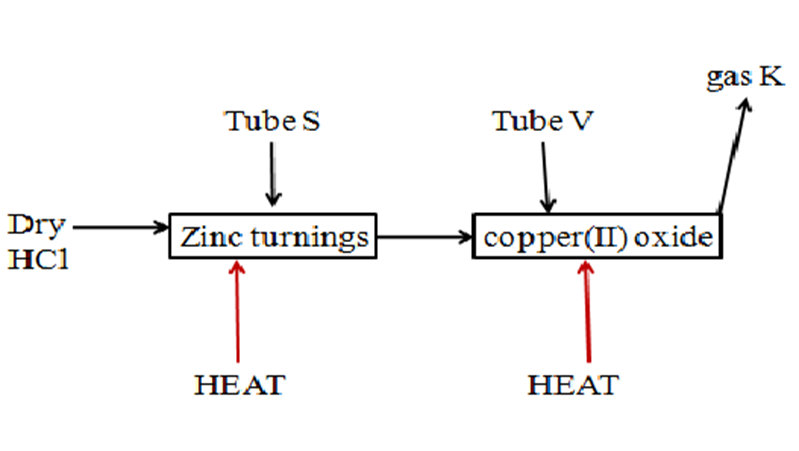
Write the equation for the reaction :
(i) for the preparation of hydrogen chloride gas. (1mk)
(ii)in tube S(1mk)
b)State and explain the observation made in tube V. (2mk)
(c)How would the total mass of tube S and tube V and their contents compare before and after the experiment.
Tube S(2mk)
Tube V(2mk)
(d)Gas K was condensed to liquid K.
(i)Identify liquid K(1mk)
(ii)Describe a simple chemical test to identify Liquid K(3mk)
(iii)A small piece of sodium metal was placed into a beaker containing liquid K.
I. State three observations made( 3mk)
II.Write an equation for the reaction that take place(1mk)
III.What is the pH of the resulting solution. Explain(2mk)
8.Using dot(.) and cross(x) to represent electrons, show the bonding in.
(a)hydroxonium ion(H3O+)(2mk)
(b)Carbon(IV)oxide(CO2)(2mk)
(c)Carbon(II)oxide(CO)(2mk)
(d)Ammonia(NH3)(2mk)
(e)Ammonium ion(NH4+)(2mk)
(f)Magnesium chloride(MgCl2)(2mk)
(g)Ethane(C2H6)(2mk)
9.Study the set- up below and answer the questions that follow
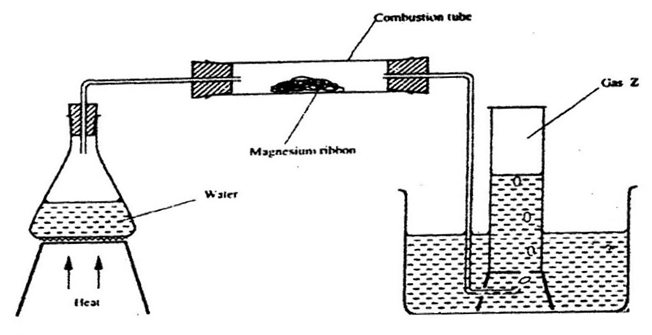
(a) Write an equation for the reaction, which take place in the combustion tube.
(b) What property of gas Z allows it to be collected as shown in the diagram
(c) State two uses of gas Z
