(i)Calculate the time taken in hours for 230kg of sodium to be produced in the Downs cell when a current of 120kA is used.
(ii)Determine the volume of chlorine released to the atmosphere. (Na=23.0),Faraday constant=96500C.I mole of a gas =24dm3 at r.t.p)
Working:
Equation at the cathode:
2Na+ (l) + 2e -> 2Na(l)
2 mole of electrons = 2 Faradays = 2 x 96500 C deposits a mass = molar mass of Na = 23.0g thus;
23.0 g -> 2 x 96500 C
(230 x 1000)g -> 230 x 1000 x 2 x 96500
23
= 1,930,000,000 / 1.93 x 10 9C
Time(t) in seconds = Quantity of electricity Current(I) in amperes
Substituting
= 1,930,000,000 / 1.93 x 10 9C
120 x 1000A
= 16,083,3333seconds / 268.0556 minutes
= 4.4676hours
Volume of Chlorine
Method 1
Equation at the anode:
2 Cl– (l) -> Cl2(g) + 2e
From the equation:
2 moles of electrons = 2 Faradays =2 x 96500C
2 x 96500C -> 24dm3
1,930,000,000 / 1.93 x 10 9C->1,930,000,000 / 1.93×10 9C x 24
2 x 96500C
Volume of Chlorine = 240,000dm3 /2.4 x 105dm3
Method 2
Equation at the anode: Cl– (l) -> Cl2(g) + 2e
Mole ratio of products at Cathode: anode = 1:1
Moles of sodium at cathode =(230 x 1000 )g= 10,000moles
23
10,000moles of Na = 10,000moles moles of Chlorine
1 moles of Chlorine gas = 24000cm3
10,000moles of Chlorine- > 10000 x 24
=240,000dm3 / 2.4x 105dm3
Method 3
Equation at the anode: Cl– (l) -> Cl2(g) + 2e
Ratio of Faradays of products at Cathode: anode = 2:2
=> 2 x 96500C produce 24000cm3 of chlorine gas Then: 1,930,000,000 / 1.93 x 10 9C ->
1,930,000,000 / 1.93 x 10 9C x24 = 240,000dm3
2 x 96500
(iij)The sodium metal produced was reacted with water to form 25000dm3 solution in a Caster-Keller tank.
(a)Calculate the concentration of the resulting solution in moles per litre.
(b)The volume of gaseous products formed at s.t.p(1 mole of gas =22.4 dm3 at s.t.p)
Chemical equation at Caster-Keller tank
2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2 (g)
Mole ratio Na:NaOH = 2 : 2 => 1:1
Moles Na =10000moles=10000moles of NaOH
25000dm3 ->10000moles of NaOH
1dm3 -> 10000 x 1 = 0.4M / 0.4 moles/dm3
25000
Mole ratio Na: H2 (g) = 2 : 1
Moles Na = 10000moles = 5000moles of H2 (g)
Volume of H2 (g) = moles x molar gas volume at s.t.p
=>5000moles x 22.4 dm3
=120,000dm3
(iv)The solution formed was further diluted with water for a titration experiment. 25.0 cm3 of the diluted solution required 20.0cm3 of 0.2M sulphuric(VI)acid for complete neutralization. Calculate the volume of water added to the diluted solution before titration.
Chemical equation
2NaOH(aq) + H2SO4(aq) -> Na2SO4(aq) + H2O(l)
Moles ratio NaOH : H2SO4 = 2 : 1
Moles ratio H2SO4 = molarity x volume => 0.2M x 20
1000 1000
=4.0 x 10-3 moles
Moles NaOH = 2 x 4.0 x 10-3 moles= 8.0 x 10-3 moles
Molarity of NaOH= Moles x 1000=> 8.0 x 10-3 moles x 1000
volume 25
=0.16 molesdm-3 /M
Volume used during dilution
C1V1 = C2V2 => 0.4M x V1 = 0.16 M x 25
= 0.16 M x 25 = 10cm3
0.4
(a) Below is a simplified diagram of the Downs Cell used for the manufacture of sodium. Study it and answer the questions that follow
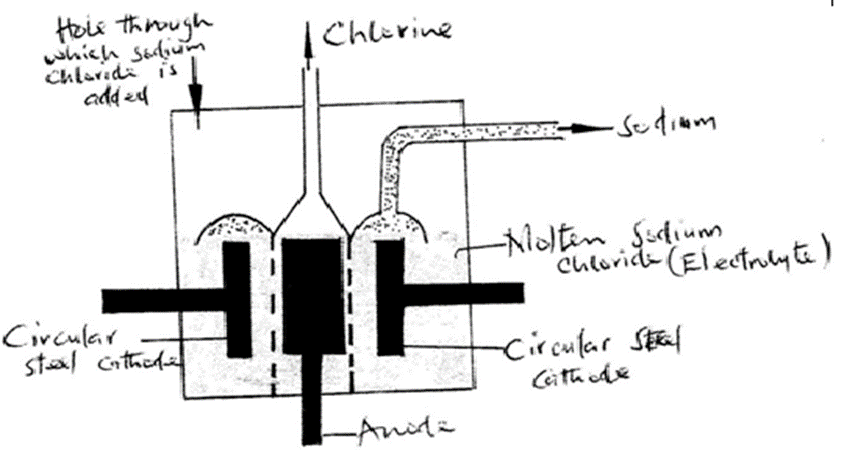
(i)What material is the anode made of? Give a reason (2 mks)
Carbon graphite/Titanium
This because they are cheap and inert/do not influence/affect the products of electrolysis
(ii) What precaution is taken to prevent chlorine and sodium from re- combination? ( 1 mks)
Using a steel gauze/diaphragm separating the cathode from anode
(iii) Write an ionic equation for the reaction in which chlorine gas is formed ( 1mk)
2Cl–(l) -> Cl2(g) + 2e
(b) In the Downs process, (used for manufacture of sodium), a certain salt is added to lower the melting point of sodium chloride from about 8000C to about 6000C.
(i) Name the salt that is added (1mk)
Calcium chloride
(ii) State why it is necessary to lower the temperature(1mk)
To reduce the cost of production
(c) Explain why aqueous sodium chloride is not suitable as an electrolyte for the manufacture of sodium in the Downs process( 2mk)
The sodium produced react explosively/vigorously with water in the aqueous sodium chloride
(d) Sodium metal reacts with air to form two oxides. Give the formulae of two oxides ( 1mk)
Na2O Sodium oxide(in limited air)
Na2O2 Sodium peroxide(in excess air)
