The rationale of studying metals cannot be emphasized.Since ages, the world over, metals like gold and silver have been used for commercial purposes.
The periodicity of alkali and alkaline earth metals was discussed in year 2 of secondary school education. This topic generally deals with:
(a)Natural occurrence of the chief ores of the most useful metals for industrial /commercial purposes.
(b)Extraction of these metals from their ores for industrial/ commercial purposes.
(c)industrial/ commercial uses of these metals.
(d)main physical and chemical properties /characteristic of the metals.
The metals given detailed emphasis here are; Sodium, Aluminium, Iron, Zinc, Lead and Copper.
| If deep on the earth’s crust deep mining is used |
The main criteria used in extraction of metals is based on its position in the electrochemical/reactivity series and its occurrence on the earth’s crust.
| Electrolysis of the ore is used for reactive metals; Potassium, Sodium, Magnesium, Calcium, Aluminium |
| The oxide is reduced using carbon/ carbon(II) oxide in a furnace if it is made of Zinc ,Tin, Lead ,Copper and Iron |
| The ore first roasted if it is a carbonate or sulphide of Zinc, Iron, Tin, Lead, and Copper to form the oxide |
| If the ore is low grade oil, water, and air is blown forming a froth(froth flotation) to concentrate |
| If near the surface, open cast mining / quarrying is used |
1.SODIUM
- Natural occurrence
Sodium naturally occurs as:
(i)Brine-a concentrated solution of sodium chloride(NaCl(aq)) in salty seas and oceans.
(ii)Rock salt-solid sodium chloride(NaCl(s)
(iii)Trona-sodium sesquicarbonate(NaHCO3.Na2CO3.2H2O) especially in lake Magadi in Kenya.
(iv)Chile saltpeter-sodium nitrate(NaNO3)
b)(i)
Extraction of Sodium from brine/Manufacture of Sodium hydroxide/The flowing mercury cathode cell/ TheCaster-Keller process
I.Raw materials
(i) Brine-concentrated solution of sodium chloride (NaCl (aq)) from salty seas and oceans.
(ii)Mercury
(iii)Water from river/lakes
II. Chemical processes
Salty lakes, seas and oceans contain large amount of dissolved sodium chloride (NaCl (aq)) solution.
This solution is concentrated to form brine which is fed into an electrolytic chamber made of suspended Carbon graphite/titanium as the anode and a continuous flow of Mercury as the cathode.Note
Mercury is the only naturally occurring known liquid metal at room
temperature and pressure
Questions
I. Write the equation for the decomposition of the electrolyte during the electrolytic process.
H2O(l) H+(aq) + OH–(aq)
NaCl(aq) Na+(aq) + Cl–(aq)
II. Name the ions present in brine that moves to the:
(i)Mercury cathode; H+(aq) , Na+(aq)
(ii)Titanium/graphite; OH–(aq), Cl–(aq)
III. Write the equation for the reaction that take place during the electrolytic process at the;
Cathode; 2Na+(aq) + 2e 2Na(s)
Anode; 2Cl–(aq) Cl2(g) + 2e
Note
(i)Concentration of 2Cl–(aq) ions is higher than OH– ions causing overvoltage thus blocking OH– ions from being discharged at the anode.
(ii)Concentration of Na+(aq) ions is higher than H+ ions causing overvoltage thus blocking H+ ions from being discharged at the cathode.
IV. Name the products of electrolysis in the flowing mercury-cathode cell.
(i)Mercury cathode; Sodium metal as grey soft metal/solid
(ii)Titanium/graphite; Chlorine gas as a pale green gas that turns moist blue/red litmus papers red then bleaches both. Chlorine gas is a very useful by-product in;
(i)making (PVC)polyvinylchloride(polychloroethene) pipes.
(ii)chlorination/sterilization of water to kill germs.
(iii)bleaching agent
(iv)manufacture of hydrochloric acid.
Sodium produced at the cathode immediately reacts with the mercury at the cathode forming sodium amalgam(NaHg) liquid that flow out of the chamber.
Na(s) + Hg(l) Na Hg (l)
Sodium amalgam is added distilled water and reacts to form sodium hydroxide solution, free mercury and Hydrogen gas.
2Na Hg (l) + 2H2O(l) 2NaOH (aq) + 2Hg(l) + H2(g)
Hydrogen gas is a very useful by-product in;
(i)making ammonia gas in the Haber process
(ii)manufacture of hydrochloric acid
(iii)in weather balloons to forecast weather
(iv)as rocket fuel
As the electrolysis of brine continues, the concentration of Cl-ions decreases and oxygen gas start being liberated. Continuous feeding of the electrolyte is therefore very necessary.
III.Uses of sodium hydroxide
The sodium hydroxide produced is very pure and is used mainly in:
(i)Making soapy and soapless detergents.
(ii)making cellulose acetate/rayon
IV. Diagram showing the Manufacture of Sodium hydroxide from the flowing Mercury-cathode cell.
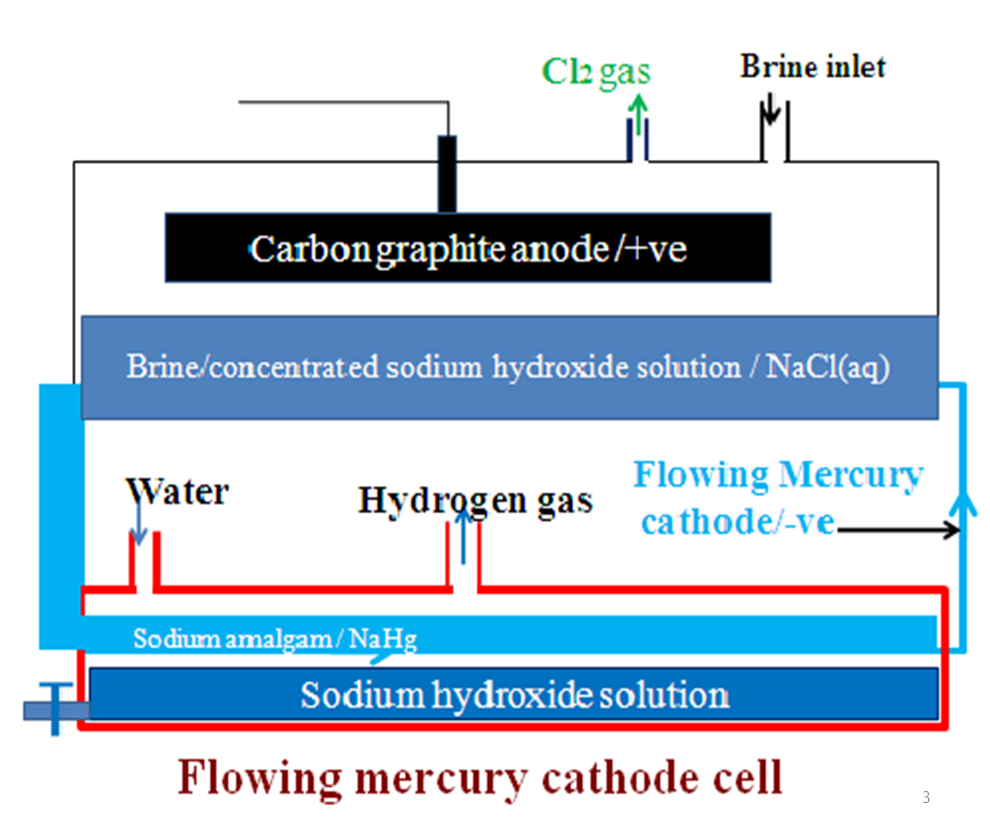
V. Environmental effects of Manufacture of Sodium hydroxide from the flowing Mercury-cathode cell.
1.Most of the Mercury used at the cathode is recycled ;
(i)to reduce the cost because mercury is expensive
(ii)to reduce pollution because mercury kills marine life.
(iii)because it causes chromosomal/genetic mutation to human beings.
2.Chlorine produced at the anode;
(i)has a pungent irritating smell that causes headache to human beings.
(ii)bleaches any wet substance.
(iii)dissolves water to form both hydrochloric acid and chloric(I)acid
Both cause marine pollution and stomach upsets.
b)(ii)
Extraction of sodium from rock salt/The Downs cell/process
I. Raw materials
(i)Rock salt/solid sodium chloride
(ii)calcium(II)chloride
II. Chemical processes.
Rock salt/ solid sodium chloride is heated to molten state in a chamber lined with fire bricks on the outside.
Sodium chloride has a melting point of about 800oC. A little calcium (II) chloride is added to lower the melting point of the electrolyte to about 600oC.
The molten electrolyte is the electrolyzed in a carbon graphite anode suspended at the centre and surrounded by steel cathode.
Questions
I. Write the equation for the decomposition of the electrolyte during the electrolytic process.
NaCl(l) Na+(l) + Cl–(l)
Note: In absence of water, the ions are in liquid state.
II. Name the ions present in molten rock salt that move to the;
(i)Steel cathode -Na+(l)
(ii)Carbon graphite anode- Cl–(l)
III. Write the equation for the reaction that take place during the electrolytic process at the;
(i)Steel cathode
2Na+(l) + 2e 2Na(l)
(ii)Carbon graphite anode
2Cl–(l) Cl2(g) + 2e
IV. Name the products of electrolysis in the Downs cell at;
(i)Cathode:
Grey solid Sodium metal is less dense than the molten electrolyte and therefore float on top of the cathode to be periodically tapped off.
(ii)Anode:
Pale green chlorine gas that turns moist/damp/wet blue/red litmus papers red then bleaches/decolorizes both. Chlorine gas is again a very useful by-product in;
(i)making (PVC)polyvinylchloride(polychloroethene) pipes.
(ii)chlorination/sterilization of water to kill germs.
(iii)bleaching agent
(iv)manufacture of hydrochloric acid.
A steel diaphragm/gauze is suspended between the electrodes to prevent recombination of sodium at the cathode and chlorine gas at the anode back to sodium chloride.
III. Diagram showing the Downs cell/process for extraction of sodium
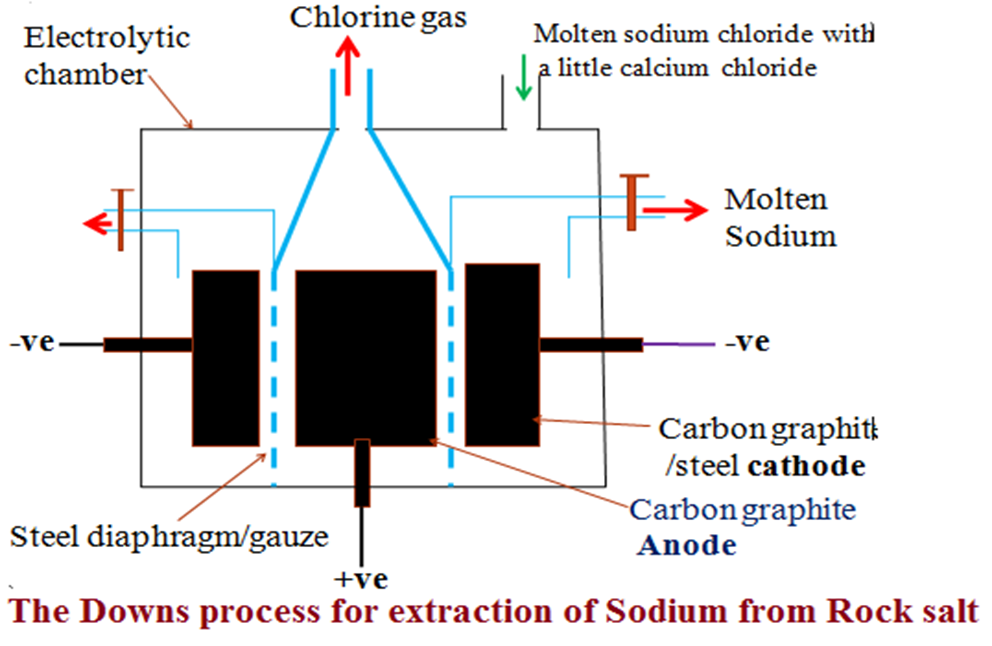
IV. Uses of sodium.
1.Sodium vapour is used as sodium lamps to give a yellow light in street lighting.
2.Sodium is used in making very useful sodium compounds like;
(i)Sodium hydroxide(NaOH)
(ii)Sodium cyanide(NaCN)
(iii)Sodium peroxide(Na2O2)
(iv)Sodamide(NaNH2)
3.An alloy of Potassium and Sodium is used as coolant in nuclear reactors.
V. Environmental effects of Downs cell.
1.Chlorine produced at the anode;
(i)has a pungent irritating smell that causes headache to human beings.
(ii)bleaches any wet substance.
(iii)dissolves water to form both hydrochloric acid and chloric(I)acid
Both cause marine pollution and stomach upsets.
2.Sodium metal rapidly react with traces of water to form alkaline Sodium hydroxide(NaOH(aq))solution. This raises the pH of rivers/lakes killing aquatic lifein case of leakages.
VI. Test for presence of Na.
If a compound has Na+ ions in solid/molten/aqueous state then it changes a non-luminous clear/colourless flame to a yellow coloration but does not burn
Experiment
Scoop a portion of sodium chloride crystals/solution in a clean metallic spatula. Introduce it to a clear /colourless Bunsen flame.
| Observation | Inference |
| Yellow coloration | Na+ |
GENERAL SUMMARY OF METALS
a) Summary methods of extracting metal from their ore
| Add oil, water, and blow air to form froth to concentrate the ore if it is a low grade |
| If near the surface use open cast mining / quarrying |
| Position on the earth’s crust |
The main criteria used in extraction of metals is based on its position in the electrochemical/reactivity series and its occurrence on the earth’s crust.
b) Summary of extraction of common metal.
| Metal | Chief ore/s | Chemical formula of ore | Method of extraction | Main equation during extraction | |
| Sodium | Rock salt | NaCl(s) | Downs process Through electrolysis of molten NaCl (CaCl2 lower m.pt from 800oC-> 600oC) | Cathode: 2Na+(l) + 2e -> 2Na(l) Anode: 2Cl–(l) -> Cl2(g) + 2e | |
| Sodium/ sodium hydroxide | Brine | NaCl(aq) | Flowing mercury cathode cell Through electrolysis of concentrated NaCl(aq) | Cathode: 2Na+(aq)+2e ->2Na(aq) Anode: 2Cl–(aq) -> Cl2(g) + 2e | |
| Aluminium | Bauxite | Al2O3.2H2O | Halls process Through electrolysis of molten Al2O3. (Cryolite lower m.pt from 2015oC -> 800oC) | Cathode: 4Al3+(l) + 12e -> 4Al(l) Anode: 6O2-(l) -> 3O2(g) + 12e | |
| Iron | Haematite Magnetite | Fe2O3 Fe3O4 | Blast furnace Reduction of the ore by carbon(II)oxide | Fe2O3(s)+ 3CO(g) 2Fe(l) +3CO2(g) Fe3O4(s)+ 4CO(g) 3Fe(l) +4CO2(g) | |
| Copper | Copper pyrites | CuFeS2 | Roasting the ore in air to get Cu2S. Heating Cu2S ore in regulated supply of air. Reduction of Cu2O by Cu2S | 2CuFeS2 (s)+ 4O2(g) -> Cu2S(s)+3SO2(g) +2FeO(s) 2Cu2S (s)+ 3O2(g) -> 2Cu2O(s)+2SO2(g) Cu2S (s)+ 2Cu2O(s) -> 6Cu(s)+ SO2(g) | |
| Zinc | Calamine | ZnCO3 | Roasting the ore in air to get ZnO Blast furnace /reduction of the oxide by Carbon(II)Oxide/Carbon | ZnCO3(s)-> ZnO(s) + CO2(g) 2ZnS(s) +3O2(g) -> 2ZnO(s) + 2SO2(g) ZnO(s) + CO(g)-> Zn(s) + CO2(g) | |
| Lead | Galena | PbS | Blast furnace-Reduction of the oxide by carbon(II)oxide /Carbon | PbO(s) + CO(g)-> Pb(s) + CO2(g) | |
of metal.
| Alloy name | Constituents of the alloy | Uses of the alloy |
| Brass | Copper and Zinc | Making scews and bulb caps |
| Bronze | Copper and Tin | Making clock springs,electrical contacts and copper coins |
| Soldier | Lead and Tin | Soldering, joining electrical contacts because of its low melting points and high thermal conductivity |
| Duralumin | Aluminium, Copper and Magnesium | Making aircraft , utensils ,windows frames because of its light weight and corrosion resistant. |
| Steel | Iron, Carbon ,Manganese and other metals | Railway lines , car bodies girders and utensils. |
| Nichrome | Nichrome and Chromium | Provide resistance in electric heaters and ovens |
| German silver | Copper,Zinc and Nickel | Making coins |
d) Physical properties of metal.
Metals form giant metallic structure joined by metallic bond from electrostatic attraction between the metallic cation and free delocalized electrons.
This makes metals to have the following physical properties:
(i)High melting and boiling points
The giant metallic structure has a very close packed metallic lattice joined by strong electrostatic attraction between the metallic cation and free delocalized electrons.The more delocalized electrons the higher the melting/boiling points e.g.
Aluminium has a melting point of about 2015oC while that of sodium is about 98oC.This is mainly because aluminium has more/three delocalized electrons than sodium/has one.
Aluminium has a boiling point of about 2470oC while that of sodium is about 890oC.This is mainly because aluminium has more/three delocalized electrons than sodium/has one.
(ii)High thermal and electrical conductivity
All metals are good thermal and electrical conductors as liquid or solids. The more delocalized electrons the higher the thermal and electrical conductivity. e.g.
Aluminium has an electrical conductivity of about 3.82 x 19-9 ohms per metre. Sodium has an electrical conductivity of about 2.18 x 19-9 ohms per metre.
(iii)Shiny/Lustrous
The free delocalized electrons on the surface of the metal absorb, vibrate and then scatter/re-emit/lose light energy. All metals are therefore usually shades of grey in colour except copper which is shiny brown.e.g.
Zinc is bluish grey while iron is silvery grey.
(iv)High tensile strength
The free delocalized electrons on the surface of the metalatoms binds the surface immediately when the metal is coiled/folded preventing it from breaking /being brittle.
(v)Malleable.
Metals can be made into thin sheet. The metallic crystal lattice on being beaten/pressed/hammered on two sides extend its length and width/bredth and is then immediately bound by the delocalized electrons preventing it from breaking/being brittle.
(vi)Ductile.
Metals can be made into thin wires. The metallic crystal lattice on being beaten/pressed/hammered on all sides extend its length is then immediately bound by the delocalized electrons preventing it from breaking/being brittle.
Revision questions
1.Given some soil , dilute sulphuric(VI)acid,mortar,pestle,filter paper,filter funnel and 2M aqueous ammonia,describe with explanation,how you would show that the soil contain Zinc.
Place the soil sample in the pestle. Crush using the mortar to reduce the particle size/increase its surface area.
Add dilute sulphuric(VI)acid to free the ions in soil sample.
Filter to separate insoluble residue from soluble filtrate
To filtrate,add three drops of aqueous ammonia as precipitating reagent. A white precipitate of Zn(OH)2, Pb(OH)2 or Al(OH)3 is formed
Add excess aqueous ammonia to the white precipitate. If it dissolves the Zn2+ ions are present. Zn(OH)2 react with excess ammonia to form soluble [Zn(OH)4]2+ complex.
2.In the extraction of aluminium,the oxide is dissolved in cryolite.
(i)What is the chemical name of cryolite?
Sodium hexafloroaluminate/Na3AlF6
(ii)What is the purpose of cryolite?
To lower the melting point of the electrolyte/Aluminium oxide from about 2015oC to 900oC
(iii)Name the substance used for similar purpose in the Down cell
Calcium chloride/CaCl2
(iv)An alloy of sodium and potassium is used as coolant in nuclear reactors.Explain.
Nuclear reactors generate a lot of heat energy. sodium and potassium alloy reduce/lower the high temperature in the reactors.
(v)Aluminium metal is used to make cooking utensils in preference to other metals.Explain.
Aluminium
(i) is a very good conductor of electricity because it has three delocalized electrons in its metallic structure
(ii)is cheap,malleable,ductile and has high tensile strength
(iii)on exposure to fire/heat form an impervious layer that prevent it from rapid corrosion.
3.Study the scheme below and use it to answer the questions that follow.
(a)Identify:
(i)solid residue L
Iron(III)Oxide/Fe2O3
(ii)Solid N
Aluminium hydroxide /Al(OH)3
(iii)Filtrate M
Sodium tetrahydroxoaluminate/ NaAl(OH)4 and sodium silicate/ NaSiO3
(iv)Solid P
Aluminium oxide/ Al2O3
(v)Gas Q
Oxygen/O2
(vi)Process K1
Filtration
(vii)Process K2
Electrolysis
(b)Write the equation for the reaction taking place in the formation of solid P from solid N
2Al(OH)3 -> Al2O3 (s) + 3H2O(l)
(c)Name a substance added to solid N before process Process K2 take place.
Cryolite/Sodium tetrahydroxoaluminate/ NaAl(OH)4
(d)State the effect of evolution of gas Q on
(i)process K2
Oxygen produced at the anode reacts with the carbon anode to form carbon(IV) oxide which escape. The electrolytic process needs continuous replacement of the carbon anode.
(ii)the environment
Oxygen produced at the anode reacts with the carbon anode to form carbon(IV) oxide which escape to the atmosphere.CO2 is a green house gas that cause global warming.
(e)An aluminium manufacturing factory runs for 24 hours. If the total mass of aluminium produced is 27000kg,
(i)Calculate the current used. (Faraday constant=96500Coulombs, Al=27.0).
(ii)assuming all the gas produced react with 200kg of anode ,calculate the loss in mass of the electrode.(Molar gas volume at room temperature = 24dm3,C=12.0)
Working
Equation at Cathode Al3+(l) + 3e -> Al(l)
27g Al -> 3 Faradays = 3 x 96500C
(27000kg x 1000) g -> (27000kg x 1000) g x 3 x 96500C 27g
=289500000000 Coulombs
Current = Quantity of electricity =>289500000000 Coulombs Time in seconds 24 x 60 x 60
3350690Ampheres
Working
Equation at Anode 2O2-(l) + 4e -> O2(g)
4 Faradays -> 4 x 96500C24dm3 O2(g) –
289500000000 Coulombs -> 289500000000 Coulombs x 24dm3 4 x 96500C
18,000,000dm3
Chemical equation at anode
O2(g) + C (s) -> CO2(g)
Method 1
24dm3 of O2(g) -> 12.0g Carbon
18,000,000dm3 ofO2(g) -> 18,000,000dm3 x 12 = 9000000g = 9000kg 24dm3 1000g
Loss in mass of the carbon graphite anode = 9000kg
NB:Mass of the carbon graphite anode remaining =27000kg – 9000kg =18000kg
The flow chart below shows the extraction of iron metal.Use it to answer the questions that follow.
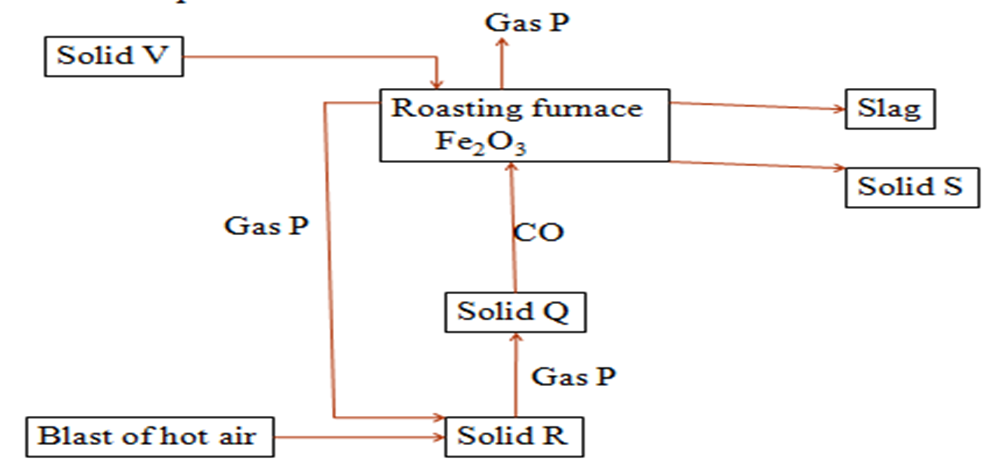
(a)Identify:
(i)gas P
Carbon(IV)oxide/CO2
(ii)Solid Q
Carbon/coke/charcoal
(iii)Solid R
Carbon/coke/charcoal
(iv)Solid V
Limestone/calcium carbonate/CaCO3
(v)Solid S
Iron/Fe
(b)Write the chemical equation for the reaction for the formation of:
(i)Solid S
Fe2O3(s) + 3CO(g) -> 2Fe(s) + 3CO2(g)
(ii)Carbon(II)oxide
C(s) + CO2 (g) -> 2CO (g)
(iii)Slag
SiO2(s) + CaO(s) -> CaSiO3(s)
Al2O3 (s) + CaO(s) -> Ca Al2O4(s)
(iv)Gas P
C(s) + O2 (g) -> CO2 (g)
(c)State two uses of:
(i)Solid S
Iron is used in making:
(i)gates ,pipes, engine blocks, rails, charcoal iron boxes, lamp posts because it is cheap.
(ii)nails, cutlery, scissors, sinks, vats, spanners, steel rods, and railway points from steel.
Steel is an alloy of iron with carbon, and/or Vanadium, Manganese, Tungsten, Nickel ,Chromium.
It does not rust/corrode like iron.
(ii)Slag
(i) tarmacing roads
(ii) cement manufacture
(iii) as building construction material
3.You are provided with sulphuric(VI)acid ,2M aqueous ammonia and two ores suspected to contain copper and iron. Describe with explanation how you would differentiate the two ores.
Crush the two ores separately in using a mortar and pestle to reduce the particle size/increase the surface area.
Add sulphuric(VI)acid to separate portion of the ore. Filter.
To a portion of the filtrate,add three drops of 2M aqueous ammonia then axcess
Results
A green precipitate insoluble in excess 2M aqueous ammonia confirms the ore contain Fe2+ ion.
A brown precipitate insoluble in excess 2M aqueous ammonia confirms the ore contain Fe3+ ion.
A blue precipitate that dissolve in excess 2M aqueous ammonia to form a deep/royal blue solution confirms the ore contain Cu2+ ion.
4. Use the flow chart below showing the extraction of Zinc metal to answer the questions that follow
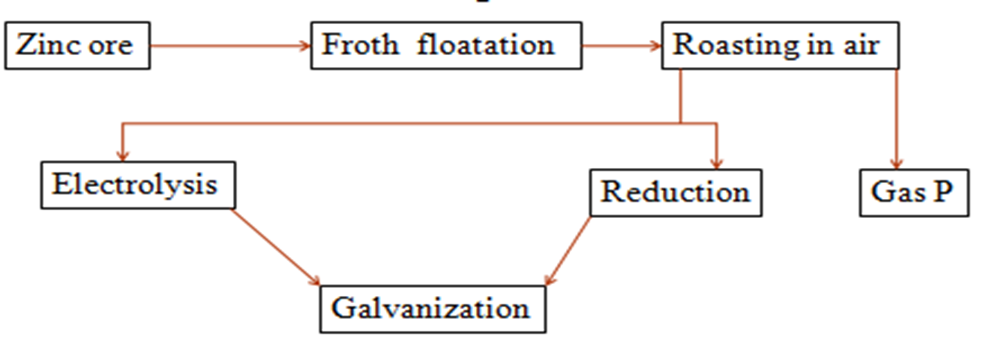
(i)two ores from which Zinc can be extracted
Calamine(ZnCO3)
Zinc blende(ZnS)
(ii)two possible identity of gas P
Sulphur(IV)oxide(SO2) from roasting Zinc blende
Carbon(IV)oxide(CO2) from decomposition of Calamine.
(b)Write a possible chemical equation taking place in the roasting chamber.
2ZnS(s) + 3O2 (g) -> 2ZnO(s) + 2SO2(g)
ZnCO3(s) -> ZnO(s) + CO2(g)
(c)Explain the effect of the by-product of the roating on the environment.
Sulphur (IV)oxide from roasting Zinc blende is an acidic gas that causes “acid rain” on dissolving in rain water.
Carbon(IV)oxide(CO2) from decomposition of Calamine is a green house gas that causes global warming.
(d)(i)Name a suitable reducing agent used in the furnace during extraction of Zinc.
Carbon(II)oxide
(ii)Write a chemical equation for the reduction process
ZnO(s) + CO(g) -> Zn(s) + CO2(g)
(e)(i)Before electrolysis, the products from roasting is added dilute sulphuric (VI)acid. Write the equation for the reaction with dilute sulphuric(VI)acid.
ZnO(s) + H2SO4 (aq) -> Zn SO4(aq) + H2(g)
(ii)During the electrolysis for extraction of Zinc,state the
I. Anode used
Aluminium sheet
II. Cathode used
Lead plate coated with silver
(ii)Write the equation for the electrolysis for extraction of Zinc at the:
I.Cathode;
Zn2+(aq) + 2e -> Zn(s)
II.Anode;
4OH–(aq) -> 2H2O(l) + O2(s) + 4e
(f)(i)What is galvanization
Dipping Iron in molten Zinc to form a thin layer of Zinc to prevent iron from rusting.
(ii)Galvanized iron sheet rust after some time. Explain
The thin layer of Zinc protect Iron from rusting through sacrificial protection. When all the Zinc has reacted with elements of air, Iron start rusting.
(g)State two uses of Zinc other than galvanization.
Making brass(Zinc/copper alloy)
Making german silver(Zinc/copper/nickel alloy)
As casing for dry cells/battery
(h)Calculate the mass of Zinc that is produced from the reduction chamber if 6400kg of Calamine ore is fed into the roaster. Assume the process is 80% efficient in each stage(Zn=64.0,C=12.0,O=16.0)
Molar mass ZnCO3(s) =124g
Molar mass Zn = 64g
Molar mass ZnO = 80g
Chemical equation
ZnCO3(s) -> ZnO(s) + CO2(g)
Method 1
124g ZnCO3 => 80g ZnO
(6400kg x1000)g ZnCO3 => (6400 x1000) x 80 = 512,000,000 g of ZnO
124
100% => 512,000,000 g of ZnO
80% => 80 x 512,000,000 g = 409600000g of ZnO
100
Chemical equation
ZnO(s) + CO(g) -> Zn(s) + CO2(g)
80g ZnO(s) => 64g Zn(s)
409600000g of ZnO => 409600000g x 64 = 327680000 g Zn
80
100% => 327680000 g Zn
80% => 80 x 327680000 g Zn = 262144000g of Zn
100
Mass of Zinc produced = 262144000g of Zn
5.An ore is suspected to bauxite. Describe the process that can be used to confirm the presence of aluminium in the ore.
Crush the ore to fine powder to increase surface area/reduce particle size.
Add hot concentrated sulphuric(VI)/nitric(V) acid to free the ions.
Filter. Retain the filtrate
Add excess aqueous ammonia to a sample of filtrate.
A white precipitate confirms presence of either Al3+ or Pb2+.
Add sodium sulphate,dilute sulphuric(VI)to another portion of filtrate.
No white precipitate confirms presence of Al3+
Or Add potassium iodide to another portion of filtrate.
No yellow precipitate confirms presence of Al3+
6.The flow chart below illustrate the industrial extraction of Lead metal
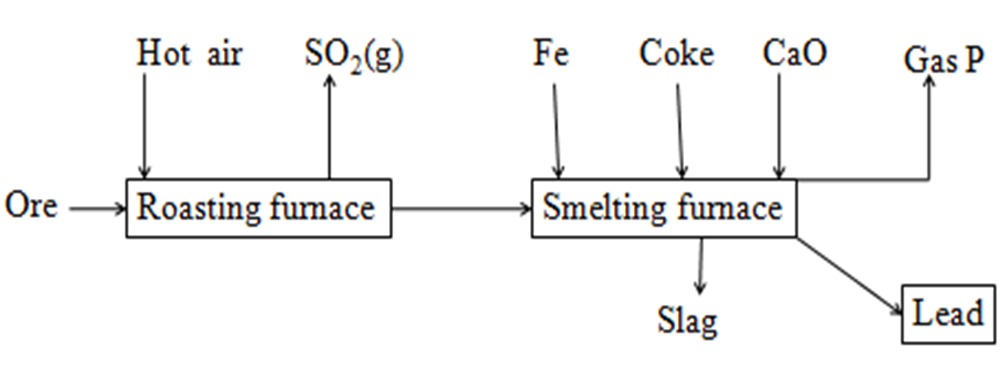
(a)(i)Name the chief ore that is commonly used in this process
Galena(PbS)
(ii)Explain what take place in the roasting furnace
