a)Natural occurrence
Aluminium is the most common naturally occurring metal. It makes 7% of the earths crust as:
(i)Bauxite ore- Hydrated aluminium oxide(Al2O3.2H2O)
(ii)Mica ore-Potassium aluminium silicate(K2Al2Si6O16)
(iii)China clay ore- aluminium silicate (Al2Si6O16)
(iv)Corrundum-Anhydrous aluminium oxide(Al2O3)
b)Extraction of aluminium from Bauxite/Halls cell/process)
The main ore from which aluminium is extracted is Bauxite ore- hydrated aluminium oxide(Al2O3.2H2O).
The ore is mined by open-caste mining method/quarrying where it is scooped together with silica/sand/silicon(IV)oxide (SiO2) and soil/ iron(III)oxide (Fe2O3) as impurities.
The mixture is first dissolved in hot concentrated sodium/potassium hydroxide solution.
The alkalis dissolve both bauxite and silicon(IV)oxide.
This is because bauxite is amphotellic while silicon(IV)oxide is acidic.
Iron(III)oxide (Fe2O3) is filtered of /removed as a residue.
Carbon(IV)oxide is bubbled into the filtrate to precipitate aluminium (III) hydroxide (Al(OH)3) as residue.
The aluminium (III) hydroxide (Al(OH)3) residue is filtered off. Silicon (IV)oxide remain in the solution as filtrate. Aluminium (III) hydroxide (Al(OH)3) residue is then heated to form pure aluminium (III)oxide(Al2O3)
2Al(OH)3 (s) Al2O3 (s) + 3H2O(l)
Pure aluminium (III)oxide (Al2O3) has a very high melting point of 2015oC.
Alot of energy is required to melt the oxide.
It is therefore dissolved first in molten cryolite /sodium hexafluoroaluminate (III)/Na3AlF6 to lower the melting point to about 800oC.
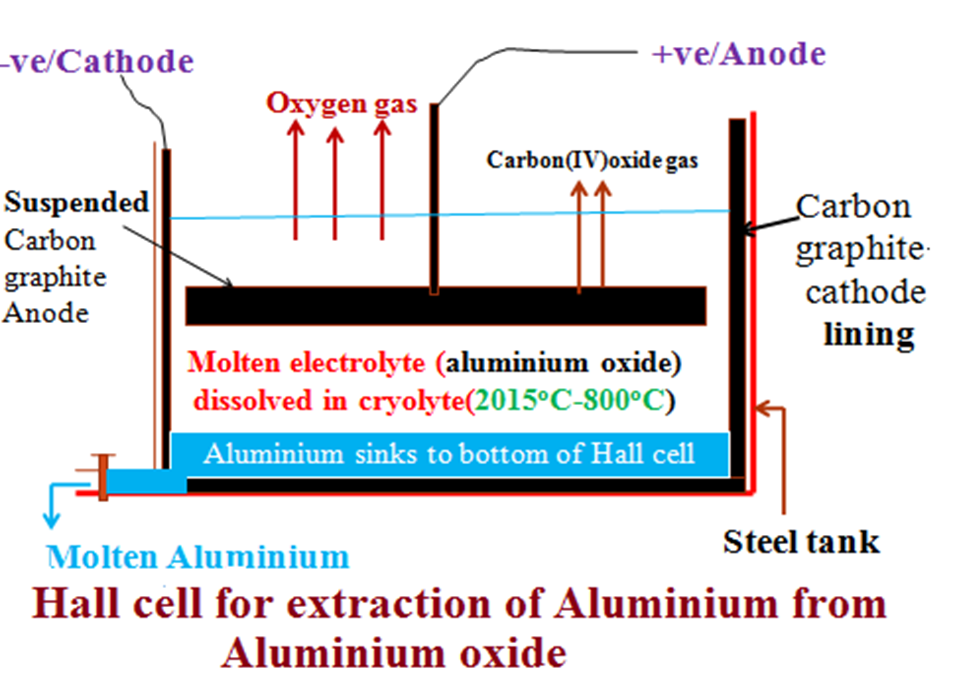
The molten electrolyte is put in the Hall cell made up of a steel tank lined with carbon graphite and an anode suspended into the electrolyte.
During the electrolysis:
(i)At the cathode;
4Al3+(l) + 12e 4Al(l)
(ii) At the anode;
6O2-(l) 3O2(g) + 12e
Aluminium is denser than the electrolyte therefore sink to the bottom of the Hall cell.
At this temperature ,the Oxygen evolved/produced at the anode reacts with carbon anode to form carbon(IV)oxide gas that escape to the atmosphere.
C(s) + O2(g) CO2(g)
The anode thus should be continuously replaced from time to time.
Flow chart summary of extraction of aluminium from Bauxite
c) Diagram showing the Hall cell / process for extraction of Bauxite
d)Uses of aluminium
(i) In making aeroplane parts, buses, tankers, furniture because aluminium is very light.
(ii)Making duralumin-an alloy which is harder and has a higher tensile strength
(iii)Making utensils,sauce pans,spoons because it is light and good conductor of electricity.
(iv)Making overhead electric cables because it is light,ductile and good conductor of electricity.
(iv)Used in the thermite process for production of Manganese, Chromium amd Titanium.
e) Environmental effects of extracting aluminium from Bauxite.
Carbon(IV)oxide gas that escape to the atmosphere is a green house gas that causes global warming.
Bauxite is extracted by open caste mining that causes soil/environmental degradation.
f) Test for presence of Al3+
If an ore is suspected to contain Al3+ it is;
(i)added hot concentrated sulphuric(VI)/Nitric(V)acid to free the ions present.
(ii)the free ions are then added a precipitating reagent like 2M sodium hydroxide /2M aqueous ammonia.
| Observation | Inference |
| White precipitate in excess 2M NaOH(aq) | Pb2+ ,Al3+, Zn2+ |
| White precipitate in excess 2M NH3(aq) | Pb2+ ,Al3+ |
| No black precipitate on adding Na2S(aq) | Al3+ |
| No white precipitate on adding either NaCl(aq),HCl(aq),H2SO4(aq),Na2SO4(aq) | Al3+ |
Practice
1.An unknown rock X was discovered in Ukraine. Test with dilute sulphuric (VI)acid shows rapid effervescence with production of a colourless gas A that forms a white precipitate with lime water and colourless solution B. On adding 3cm3 of 2M sodium hydroxide, a white precipitate C is formed that dissolves to form a colourless solution D on adding more sodium hydroxide. On adding 2M aqueous ammonia, a white precipitate E is formed which persist in excess aqueous ammonia.On which on adding 5cm3 of 1M Lead(II)nitrate(V) to F a white precipitate G is formed which remains on heating.
Identify:
A Hydrogen/H2
B Aluminium sulphate(VI)/Al2(SO4) 3
C Aluminium hydroxide/ Al(OH4) 3
D Tetrahydroxoaluminate(III)/ [Al(OH4) 3] –
E Aluminium hydroxide/ Al(OH) 3
F Aluminium chloride/ AlCl3
2.Aluminium is obtained from the ore with the formula Al2O3. 2H2O. The ore is first heated and refined to obtain pure aluminium oxide (Al2O3). The oxide is then electrolysed to get Aluminium and oxygen gas using carbon anodes and carbon as cathode. Give the common name of the ore from where aluminium is extracted from ½ mark
What would be the importance of heating the ore first before refining it?1 mark
To remove the water of crystallization
The refined ore has to be dissolved in cryolite first before electrolysis. Why is this necessary? 1½ mark
To lower the melting point of aluminium oxide from about 2015oC to 900oC so as to lower /reduce cost of production
Why are the carbon anodes replaced every now and then in the cell for electrolysing aluminium oxide? 1 mark
Oxygen produced at anode react with carbon to form carbon(IV)oxide gas that escape
State two uses of aluminium
In making aeroplane parts, buses, tankers, utensils, sauce pans,spoons
Making overhead electric cables
Making duralumin
