a)Natural occurrence
Iron is the second most common naturally occurring metal. It makes 4% of the earths crust as:
(i)Haematite(Fe2O3)
(ii)Magnetite(Fe3O4)
(iii)Siderite(FeCO3)
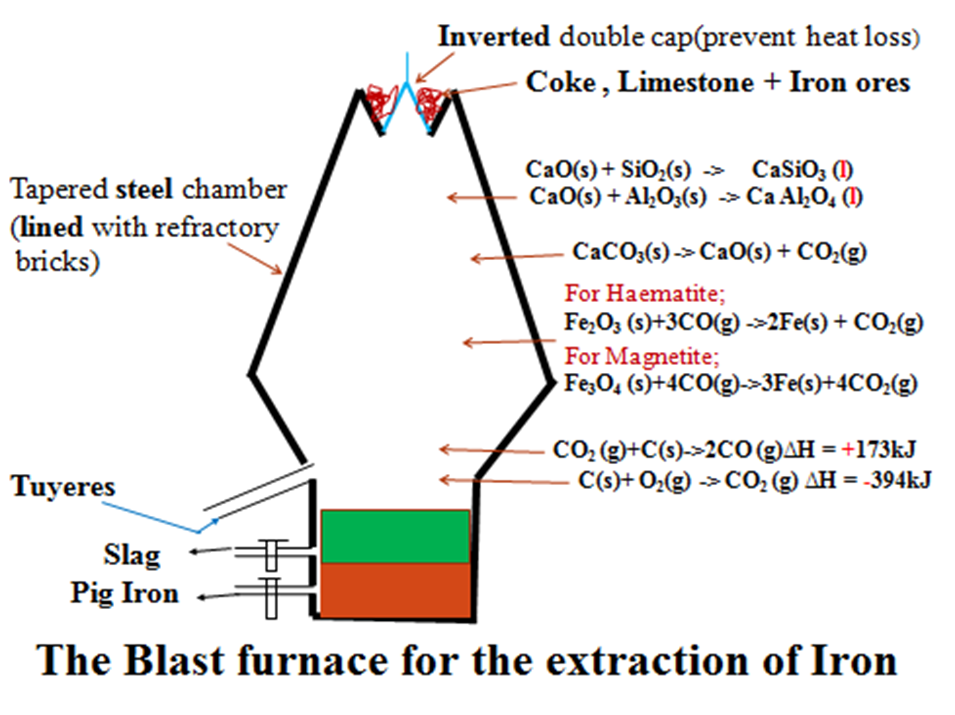
b)The blast furnace for extraction of iron from Haematite and Magnetite
a)Raw materials:
(i)Haematite(Fe2O3)
(ii)Magnetite(Fe3O4)
(iii)Siderite(FeCO3)
(iv)Coke/charcoal/ carbon
(v)Limestone
b)Chemical processes:
Iron is usually extracted from Haematite (Fe2O3), Magnetite(Fe3O4) Siderite (FeCO3).These ores contain silicon(IV)oxide(SiO2) and aluminium(III)oxide (Al2O3) as impurities.
When extracted from siderite, the ore must first be roasted in air to decompose the iron(II)Carbonate to Iron(II)oxide with production of carbon(IV)oxide gas:
FeCO3(s) FeO(s) + CO2(g)
Iron(II)oxide is then rapidly oxidized by air to iron(III)oxide(Haematite).
4FeO(s) + O2(g) 2Fe2O3(s)
Haematite (Fe2O3), Magnetite(Fe3O4), coke and limestone are all then fed from top into a tall (about 30metres in height) tapered steel chamber lined with refractory bricks called a blast furnace.
The furnace is covered with inverted double cap to prevent/reduce amount of any gases escaping .
Near the base/bottom, blast of hot air at about 1000K (827oC) is driven/forced into the furnace through small holes called Tuyeres.
As the air enters ,it reacts with coke/charcoal/carbon to form carbon(IV)oxide gas. This reaction is highly exothermic.
C(s)+ O2(g) CO2 (g) ∆H = -394kJ
This raises the temperature at the bottom of the furnace to about 2000K(1650oC).As Carbon(IV)oxide gas rises up the furnace it reacts with more coke to form carbon(II)oxide gas.This reaction is endothermic.
CO2 (g) + C(s) 2CO (g) ∆H = +173kJ
Carbon(II)oxide gas is a strong reducing agent that reduces the ores at the upper parts of the furnace where temperatures are about 750K(500oC) i.e.
For Haematite;
Fe2O3 (s) + 3CO(g) 2Fe(s) + CO2(g)
For Magnetite;
Fe3O4 (s) + 4CO(g) 3Fe(s) + 4CO2(g)
Iron is denser than iron ore. As it falls to the hotter base of the furnace it melts and can easily be tapped off.
Limestone fed into the furnace decomposes to quicklime/calcium oxide and produce more carbon(IV)oxide gas.
CaCO3(s) CaO(s) + CO2(g)
Quicklime/calcium oxide reacts with the impurities silicon(IV)oxide(SiO2) and aluminium(III)oxide(Al2O3)in the ore to form calcium silicate and calcium aluminate.
CaO(s) + SiO2(s) CaSiO3 (l)
CaO(s) + Al2O3(s) Ca Al2O4 (l)
Calcium silicate and calcium aluminate mixture is called slag.Slag is denser than iron ore but less dense than iron therefore float on the pure iron. It is tapped at different levels to be tapped off for use in:
(i)tarmacing roads
(ii) cement manufacture
(iii)as building construction material
(c)Uses of Iron
Iron obtained from the blast furnace is hard and brittle. It is called Pig iron. It is remelted, added scrap steel then cooled. This iron is called cast iron.
Iron is mainly used to make:
(i)gates ,pipes, engine blocks, rails, charcoal iron boxes,lamp posts because it is cheap.
(ii)nails, cutlery, scissors, sinks, vats, spanners,steel rods, and railway points from steel.
Steel is an alloy of iron with carbon, and/or Vanadium, Manganese, Tungsten, Nickel ,Chromium. It does not rust/corrode like iron.
e) Environmental effects of extracting Iron from Blast furnace
(i)Carbon(IV)oxide(CO2) gas is a green house gas that causes/increases global warming if allowed to escape/leak from the furnace.
(ii)Carbon(II)oxide(CO)gas is a highly poisonous/toxic odourless gas that can kill on leakage.
It is preferentially absorbed by the haemoglobin in mammals instead of Oxygen to form a stable compound that reduce free hemoglobin in the blood.
(iii) Haematite (Fe2O3), Magnetite(Fe3O4) and Siderite (FeCO3) are extracted through quarrying /open cast mining that cause soil / environmental degradation .
f) Test for the presence of Iron
Iron naturally exist in its compound as Fe2+ /Fe3+
If an ore is suspected to contain Fe2+ /Fe3+ it is;
(i)added hot concentrated sulphuric(VI)/Nitric(V)acid to free the ions present.
(ii)the free ions are then added a precipitating reagent like 2M sodium hydroxide /2M aqueous ammonia which forms;
I) an insoluble green precipitate in excess of 2M sodium hydroxide /2M aqueous ammonia if Fe2+ ions are present.
I) an insoluble brown precipitate in excess of 2M sodium hydroxide /2M aqueous ammonia if Fe2+ ions are present.
| Observation | Inference |
| green precipitate in excess 2M NaOH(aq) | Fe2+ |
| green precipitate in excess 2M NH3(aq) | Fe2+ |
| brown precipitate in excess 2M NaOH(aq) | Fe3+ |
| brown precipitate in excess 2M NH3(aq) | Fe3+ |
