(a)Calculate the solubility of potassium nitrate(V) if 5.0 g of the salt is dissolved in 50.0cm3 of water.
Solubility = Mass of solute/salt/solid x 100 =>( 5.0 x 100 ) = 10.0 g /100g H2O
Mass/volume of water/solvent 50.0
(b)Calculate the solubility of potassium chlorate(V) if 50.0 g of the salt is dissolved in 250.0cm3 of water.
Solubility = Mass of solute/salt/solid x 100 =>( 50.0 x 100 ) = 20.0 g /100g H2O
Mass/volume of water/solvent 250.0
(c)If the solubility of potassium chlorate(V) is 5g/100g H2O at 80oC,how much can dissolve in 5cm3 of water at 80oC .
Mass of solute/salt/solid = Solubility x Mass/volume of water/solvent
100
=> 5 x 5 = 0.25g of KClO3 dissolve
100
(d)If the solubility of potassium chlorate(V) is 72g/100g H2O at 20oC,how much can saturate 25g of water at 20oC .
Mass of solute/salt/solid = Solubility x Mass/volume of water/solvent
100
=> 72 x 25 = 18.0g of KClO3 dissolve/saturate
100
(e) 22g of potassium nitrate(V) was dissolved in 40.0g of water at 10oC. Calculate the solubility of potassium nitrate(V) at 10oC.
Solubility = Mass of solute/salt/solid x 100 =>( 22 x 100 ) = 55.0 g /100g H2O
Mass/volume of water/solvent 40.0.
(f)What volume of water should be added to 22.0g of water at 10oC if the solubility of KNO3 at 10oC is 5.0g/100g H2O?
Solubility is mass/100g H2O => 22.0g + x = 100cm3/100g H2O
X= 100 – 22 = 78 cm3 of H2O
2a. A graph of solubility against temperature is called solubility curve.
It shows the influence of temperature on solubility of different substances/solids/salts.
Some substances dissolve more with increase in temperature while for others dissolve less with increase in temperature
Note:
(i)solubility of KNO3 and KClO3 increase with increase in temperature.
(ii)solubility of KNO3 is always higher than that of KClO3 at any specified temperature.
(iii)solubility of NaCl decrease with increase in temperature.
(iv)NaCl has the highest solubility at low temperature while KClO3 has the lowest solubility at low temperature.
(v)At point A both NaCl and KNO3 are equally soluble.
(vi)At point B both NaCl and KClO3 are equally soluble.
(vii) An area above the solubility curve of the salt shows a saturated /supersaturated solution.
(viii) An area below the solubility curve of the salt shows an unsaturated solution.
b) For salts whose solubility increases with increase in temperature, crystals form when the salt solution at higher temperatures is cooled to a lower temperature.
- For salts whose solubility decreases with increase in temperature, crystals form when the salt solution at lower temperatures is heated to a higher temperature.
The examples below shows determination of the mass of crystals deposited with changes in temperature.
2.The solubility of KClO3 at 100oC is 60g/100g water .What mass of KClO3 will be deposited at:
(i)75 oC if the solubility is now 39g/100g water.
At 100oC = 60.0g
Less at 75oC = – 39.0g
Mass of crystallized out 21.0g
(i)35 oC if the solubility is now 28 g/100g water.
At 100oC = 60.0g
Less at 35oC = – 28.0.0g
Mass of crystallized out 32.0g
3. KNO3 has a solubility of 42 g/100g water at 20oC.The salt was heated and added 38g more of the solute which dissolved at100oC. Calculate the solubility of KNO3 at 100oC.
Solubility of KNO3 at 100oC = solubility at 20oC + mass of KNO3 added
=> 42g + 38g = 80g KNO3 /100g H2O
4. A salt solution has a mass of 65g containing 5g of solute. The solubility of this salt is 25g per 100g water at 20oC. 60g of the salt are added to the solution at 20oC.Calculate the mass of the solute that remain undissolved.
Mass of solvent at 20oC = mass of solution – mass of solute
=> 65 – 5 = 60g
Solubility before adding salt = mass of solute x 100
Volume of solvent
=> 5 x 100 = 8.3333g/100g water
60
Mass of solute to equalize with solubility = 25 – 8.3333g = 16.6667g
Mass of solute undissolved = 60.0 – 16.6667g = 43.3333 g
5. Study the table below
| Salt | Solubility in gram at | |
| 50oC | 20oC | |
| KNO3 | 90 | 30 |
| KClO3 | 20 | 6 |
(i)What happens when the two salts are dissolved in water then cooled from 50oC to 20oC.
(90 – 30) = 60.0 g of KNO3 crystals precipitate
(20 – 6) = 14.0 g of KClO3 crystals precipitate
(ii)State the assumption made in (i) above.
Solubility of one salt has no effect on the solubility of the other.
6. 10.0 g of hydrated potassium carbonate (IV) K2CO3.xH2O on heating leave 7.93 of the hydrate.
(a)Calculate the mass of anhydrous salt obtained.
Hydrated on heating leave anhydrous = 7.93 g
(b)Calculate the mass of water of crystallization in the hydrated salt
Mass of water of crystallization = hydrated – anhydrous
=> 10.0 – 7.93 = 2.07 g
(c)How many moles of anhydrous salt are there in 10of hydrate? (K= 39.0,C=12.0.O= 16.0)
Molar mass K2CO3= 138
Moles K2CO3 = mass of K2CO3 => 7.93 = 0.0515 moles
Molar mass K2CO3 138
(d)How many moles of water are present in the hydrate for every one mole of K2CO3 ? (H=1.0.O= 16.0)
Molar mass H2O = 18
Moles H2O = mass of H2O => 2.07 = 0.115 moles
Molar mass H2O 18
Mole ratio H2O : K2CO3 = 0.115 moles 2 = 2
0.0515 moles 1
(e)What is the formula of the hydrated salt?
K2CO3 .2 H2O
7. The table below shows the solubility of Potassium nitrate(V) at different temperatures.
| Temperature(oC) | 5.0 | 10.0 | 15.0 | 30.0 | 40.0 | 50.0 | 60.0 |
| mass KNO3/ 100g water | 15.0 | 20.0 | 25.0 | 50.0 | 65.0 | 90.0 | 120.0 |
(a)Plot a graph of mass of in 100g water(y-axis) against temperature in oC
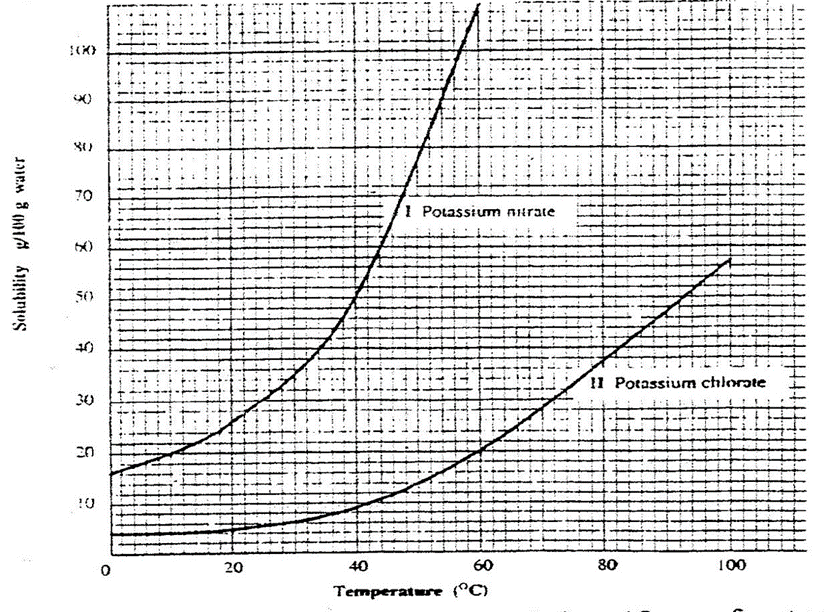
(b)From the graph show and determine
(i)the mass of KNO3 dissolved at:
I. 20oC
From a correctly plotted graph = 32g
II. 35oC
From a correctly plotted graph = 57g
III. 55oC
From a correctly plotted graph = 104g
(ii)the temperature at which the following mass of KNO3 dissolved:
I. 22g
From a correctly plotted graph =13.0oC
II. 30g
From a correctly plotted graph =17.5oC
III.100g
From a correctly plotted graph =54.5oC
(c)Explain the shape of your graph.
Solubility of KNO3increase with increase in temperature/More KNO3 dissolve as temperature rises.
(d)Show on the graph the supersaturated and unsaturated solutions.
Above the solubility curve write; “supersaturated”
Below the solubility curve write; “unsaturated”
(e)From your graph, calculate the amount of crystals obtained when a saturated solution of KNO3 containing 180g of the salt is cooled from 80oC to:
I. 20oC
Solubility before heating = 180 g
Less Solubility after heating(from the graph) = 32 g
Mass of KNO3crystals = 148 g
II. 35oC
Solubility before heating = 180 g
Less Solubility after heating(from the graph) = 58 g
Mass of KNO3crystals = 122 g
III. 55oC
Solubility before heating = 180 g
Less Solubility after heating(from the graph) = 102 g
Mass of KNO3crystals = 78 g
7. The table below shows the solubility of salts A and B at various temperatures.
| Temperature(oC) | 0.0 | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 | 70.0 | 80.0 |
| Solubility of A | 28.0 | 31.0 | 34.0 | 37.0 | 40.0 | 43.0 | 45.0 | 48.0 | 51.0 |
| Solubility of B | 13.0 | 21.0 | 32.0 | 46.0 | 64.0 | 85.0 | 110.0 | 138.0 | 169.0 |
8. (a)On the same axis plot a graph of solubility (y-axis) against temperature for each salt.
(b)At what temperature are the two salts equally soluble.
The point of intersection of the two curves = 24oC
(c)What happens when a mixture of 100g of salt B with 100g if water is heated to 80oC
From the graph, the solubility of B at 80oC is 169g /100g water. All the 100g crystals of B dissolve.
(d)What happens when the mixture in (c) above is then cooled from 50oC to 20oC.
Method I.
Total mass before cooling at 50oC = 100.0 g
(From graph) Solubility/mass after cooling at 20oC = 32.0 g
Mass of crystals deposited 68.0 g
Method II.
Mass of soluble salt crystals at 50oC added = 100 g
(From graph)Solubility/mass before cooling at 50oC = 85.0 g
Mass of crystals that cannot dissolve at 50oC 15.0 g
(From graph) Solubility/mass before cooling at 50oC = 85.0 g
(From graph) Solubility/mass after cooling at 20oC = 32.0 g
Mass of crystals deposited after cooling 53.0 g
Total mass of crystals deposited = 15.0 + 53.0 = 68.0 g
(e)A mixture of 40g of A and 60g of B is added to 10g of water and heated to 70oC.The solution is then allowed to cool to 10oC.Describe clearly what happens.
I.For salt A
Solubility of A before heating = mass of A x 100
Volume of water added
=> 40 x 100 = 400g/100g Water
10
(Theoretical)Solubility of A before heating = 400 g
Less (From graph ) Solubility of A after heating at 70oC = 48g
Mass of crystals that can not dissolve at70oC = 352 g
(From graph ) Solubility of A after heating at 70oC = 48g
Less (From graph ) Solubility of A after cooling to 10oC = 31g
Mass of crystals that crystallize out on cooling to10oC = 17 g
Mass of crystals that can not dissolve at70oC = 352 g
Add Mass of crystals that crystallize out on cooling to10oC = 17 g
Total mass of A that does not dissolve/crystallize/precipitate = 369 g
I.For salt B
Solubility of B before heating = mass of B x 100
Volume of water added
=> 60 x 100 = 600g/100g Water
10
(Theoretical)Solubility of B before heating = 600 g
Less (From graph ) Solubility of B after heating at 70oC = 138g
Mass of crystals that cannot dissolve at70oC = 462 g
(From graph ) Solubility of B after heating at 70oC = 138g
Less (From graph ) Solubility of B after cooling to 10oC = 21g
Mass of crystals that crystallize out on cooling to10oC = 117 g
Mass of crystals that cannot dissolve at70oC = 462 g
Add Mass of crystals that crystallize out on cooling to10oC = 117 g
Total mass of A that does not dissolve/crystallize/precipitate = 579 g
(f)State the assumption made in (e)above
Solubility of one salt has no effect on the solubility of the other
8. When 5.0 g of potassium chlorate (V) was put in 10cm3 of water and heated, the solid dissolves. When the solution was cooled , the temperature at which crystals reappear was noted. Another 10cm3 of water was added and the mixture heated to dissolve then cooled for the crystals to reappear .The table below shows the the results obtained
| Total volume of water added(cm3) | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 |
| Mass of KClO3 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Temperature at which crystals appear | 80.0 | 65.0 | 55.0 | 45.0 | 30.0 |
| Solubility of KclO3 | 50.0 | 25.0 | 16.6667 | 12.5 | 10.0 |
(a)Complete the table to show the solubility of KclO3 at different temperatures.
(b)Plot a graph of mass of KClO3 per 100g water against temperature at which crystals form.
(c)From the graph, show and determine ;
(i)the solubility of KClO3 at
I. 50oC
From a well plotted graph = 14.5 g KClO3/100g water
II. 35oC
From a well plotted graph = 9.0 g KclO3/100g water
(ii)the temperature at which the solubility is:
I.10g/100g water
From a well plotted graph = 38.0 oC
II.45g/100g water
From a well plotted graph = 77.5 oC
(d)Explain the shape of the graph.
Solubility of KClO3 increase with increase in temperature/more KclO3dissolve as temperature rises.
(e)What happens when 100g per 100g water is cooled to 35.0 oC
Solubility before heating = 100.0
(From the graph) Solubility after cooling = 9.0
Mass of salt precipitated/crystallization = 91.0 g
9. 25.0cm3 of water dissolved various masses of ammonium chloride crystals at different temperatures as shown in the table below.
| Mass of ammonium chloride(grams) | 4.0 | 4.5 | 5.5 | 6.5 | 9.0 |
| Temperature at which solid dissolved(oC) | 30.0 | 50.0 | 70.0 | 90.0 | 120.0 |
| Solubility of NH4Cl | 16.0 | 18.0 | 22.0 | 26.0 | 36.0 |
(a)Complete the table
(b)Plot a solubility curve
(c)What happens when a saturated solution of ammonium chloride is cooled from 80oC to 40oC.
(From the graph )Solubility at 80oC = 24.0 g
Less (From the graph )Solubility at 40oC = 16.8 g
Mass of crystallized/precipitated = 7.2 g
9. Solubility and solubility curves are therefore used
(i) to know the effect of temperature on the solubility of a salt
(ii)to fractional crystallize two soluble salts by applying their differences in solubility at different temperatures.
(iii)determine the mass of crystal that is obtained from crystallization.
10.Natural fractional crystallization takes place in Kenya/East Africa at:
(i) Lake Magadi during extraction of soda ash(Sodium carbonate) from Trona(sodium sesquicarbonate) (ii) Ngomeni near Malindi at the Indian Ocean Coastline during the extraction of common salt(sodium chloride).
Solubility and solubility curves are therefore used
(i) to know the effect of temperature on the solubility of a salt
(ii)to fractional crystallize two soluble salts by applying their differences in solubility at different temperatures.
(iii)determine the mass of crystal that is obtained from crystallization.
In an experiment, soap solution was added to three separate samples of water. The table below shows the volumes of soap solution required to form lather with 1000cm3 of each sample of water before and after boiling.
| Sample I | Sample II | Sample III | |
| Volume of soap before water is boiled (cm3) | 27.0 | 3.0 | 10.0 |
| Volume of soap after water is boiled(cm3) | 27.0 | 3.0 | 3.0 |
a) Which water sample is likely to be soft? Explain. (2mks)
Sample II: Uses little sample of soap .
c) Name the change in the volume of soap solution used in sample III (1mk)
On heating the sample water become soft bcause it is temporary hard.
2.Study the scheme below and use it to aanswer the questions that follow:
(a)Write the formula of:
(i)Cation in solution K
Al3+
(ii)white ppt L
Al(OH)3
(iii) colourless solution M
[Al(OH)4]–
(iv) colourless solution N
AlCl3
(v)white ppt P
Al(OH)3
(b)Write the ionic equation for the reaction for the formation of:
(i)white ppt L
Al3+(aq) + 3OH– (aq) -> Al(OH)3(s)
(v)white ppt P
Al3+(aq) + 3OH– (aq) -> Al(OH)3(s)
(c)What property is illustrated in the formation of colourless solution M and N.
Amphotellic
